Optogenetic targeting of cortical astrocytes selectively improves NREM sleep in an Alzheimer's disease mouse model
- PMID: 39362954
- PMCID: PMC11450172
- DOI: 10.1038/s41598-024-73082-8
Optogenetic targeting of cortical astrocytes selectively improves NREM sleep in an Alzheimer's disease mouse model
Abstract
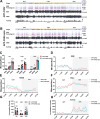
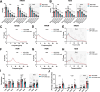
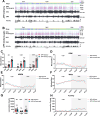
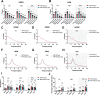
Alzheimer's disease (AD) is a progressive neurodegenerative condition marked by memory impairments and distinct histopathological features such as amyloid-beta (Aβ) accumulations. Alzheimer's patients experience sleep disturbances at early stages of the disease. APPswe/PS1dE9 (APP) mice exhibit sleep disruptions, including reductions in non-rapid eye movement (NREM) sleep, that contribute to their disease progression. In addition, astrocytic calcium transients associated with a sleep-dependent brain rhythm, slow oscillations prevalent during NREM sleep, are disrupted in APP mice. However, at present it is unclear whether restoration of circuit function by targeting astrocytic activity could improve sleep in APP mice. To that end, APP mice expressing channelrhodopsin-2 (ChR2) targeted to astrocytes underwent optogenetic stimulation at the slow oscillation frequency. Optogenetic stimulation of astrocytes significantly increased NREM sleep duration but not duration of rapid eye movement (REM) sleep. Optogenetic treatment increased delta power and reduced sleep fragmentation in APP mice. Thus, optogenetic activation of astrocytes increased sleep quantity and improved sleep quality in an AD mouse model. Astrocytic activity provides a novel therapeutic avenue to pursue for enhancing sleep and slowing AD progression.
Keywords: Alzheimer’s disease; Astrocyte; Delta power; EEG; NREM; Optogenetics; REM; Sleep; Sleep fragmentation; Slow oscillations; Slow waves; Wake.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

