Non-contact photoacoustic imaging with a silicon photonics-based Laser Doppler Vibrometer
- PMID: 39362973
- PMCID: PMC11450003
- DOI: 10.1038/s41598-024-74266-y
Non-contact photoacoustic imaging with a silicon photonics-based Laser Doppler Vibrometer
Abstract
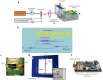
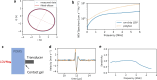
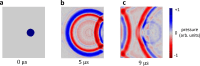
Photoacoustic imaging has emerged as a powerful, non-invasive modality for various biomedical applications. Conventional photoacoustic systems require contact-based ultrasound detection and expensive, bulky high-power lasers for the excitation. The use of contact-based detectors involves the risk of contamination, which is undesirable for most biomedical applications. While other non-contact detection methods can be bulky, in this paper, we demonstrate a proof-of-concept experiment for compact and contactless detection of photoacoustic signals on silicone samples embedded with ink-filled channels. A silicon photonics-based Laser Doppler Vibrometer (LDV) detects the acoustic waves excited by a compact pulsed laser diode. By scanning the LDV beam over the surface of the sample, 2D photoacoustic images were reconstructed of the sample.
Keywords: Laser Doppler Vibrometer (LDV); Photoacoustic imaging; Remote detection; Silicon Photonics.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Xu, M. & Wang, L. V. Photoacoustic imaging in biomedicine. Review of Scientific Instruments77, 041101. 10.1063/1.2195024 (2006). - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

