Persistence of post-stress blood pressure elevation requires activation of astrocytes
- PMID: 39363030
- PMCID: PMC11450218
- DOI: 10.1038/s41598-024-73345-4
Persistence of post-stress blood pressure elevation requires activation of astrocytes
Abstract
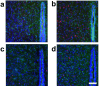
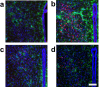
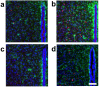
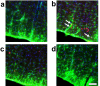
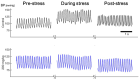
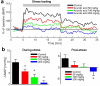
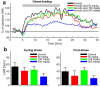
The reflexive excitation of the sympathetic nervous system in response to psychological stress leads to elevated blood pressure, a condition that persists even after the stress has been alleviated. This sustained increase in blood pressure, which may contribute to the pathophysiology of hypertension, could be linked to neural plasticity in sympathetic nervous activity. Given the critical role of astrocytes in various forms of neural plasticity, we investigated their involvement in maintaining elevated blood pressure during the post-stress phase. Specifically, we examined the effects of arundic acid, an astrocytic inhibitor, on blood pressure and heart rate responses to air-jet stress. First, we confirmed that the inhibitory effect of arundic acid is specific to astrocytes. Using c-Fos immunohistology, we then observed that psychological stress activates neurons in cardiovascular brain regions, and that this stress-induced neuronal activation was suppressed by arundic acid pre-treatment in rats. By evaluating astrocytic process thickness, we also confirmed that astrocytes in the cardiovascular brain regions were activated by stress, and this activation was blocked by arundic acid pre-treatment. Next, we conducted blood pressure measurements on unanesthetized, unrestrained rats. Air-jet stress elevated blood pressure, which remained high for a significant period during the post-stress phase. However, pre-treatment with arundic acid, which inhibited astrocytic activation, suppressed stress-induced blood pressure elevation both during and after stress. In contrast, arundic acid had no significant impact on heart rate. These findings suggest that both neurons and astrocytes play integral roles in stress-induced blood pressure elevation and its persistence after stress, offering new insights into the pathophysiological mechanisms underlying hypertension.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

