Exome sequencing of 20,979 individuals with epilepsy reveals shared and distinct ultra-rare genetic risk across disorder subtypes
- PMID: 39363051
- PMCID: PMC11646479
- DOI: 10.1038/s41593-024-01747-8
Exome sequencing of 20,979 individuals with epilepsy reveals shared and distinct ultra-rare genetic risk across disorder subtypes
Abstract
Identifying genetic risk factors for highly heterogeneous disorders such as epilepsy remains challenging. Here we present, to our knowledge, the largest whole-exome sequencing study of epilepsy to date, with more than 54,000 human exomes, comprising 20,979 deeply phenotyped patients from multiple genetic ancestry groups with diverse epilepsy subtypes and 33,444 controls, to investigate rare variants that confer disease risk. These analyses implicate seven individual genes, three gene sets and four copy number variants at exome-wide significance. Genes encoding ion channels show strong association with multiple epilepsy subtypes, including epileptic encephalopathies and generalized and focal epilepsies, whereas most other gene discoveries are subtype specific, highlighting distinct genetic contributions to different epilepsies. Combining results from rare single-nucleotide/short insertion and deletion variants, copy number variants and common variants, we offer an expanded view of the genetic architecture of epilepsy, with growing evidence of convergence among different genetic risk loci on the same genes. Top candidate genes are enriched for roles in synaptic transmission and neuronal excitability, particularly postnatally and in the neocortex. We also identify shared rare variant risk between epilepsy and other neurodevelopmental disorders. Our data can be accessed via an interactive browser, hopefully facilitating diagnostic efforts and accelerating the development of follow-up studies.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
Figures




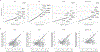





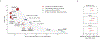
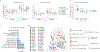

Update of
-
Exome sequencing of 20,979 individuals with epilepsy reveals shared and distinct ultra-rare genetic risk across disorder subtypes.medRxiv [Preprint]. 2024 Sep 20:2023.02.22.23286310. doi: 10.1101/2023.02.22.23286310. medRxiv. 2024. Update in: Nat Neurosci. 2024 Oct;27(10):1864-1879. doi: 10.1038/s41593-024-01747-8. PMID: 36865150 Free PMC article. Updated. Preprint.
Comment in
-
Epilepsy Gene Discovery Needs Another Big Leap Forward.Epilepsy Curr. 2025 Apr 1;25(3):201-202. doi: 10.1177/15357597251325685. eCollection 2025 May-Jun. Epilepsy Curr. 2025. PMID: 40190791 Free PMC article. No abstract available.
References
-
- World Health Organization. Epilepsy: a public health imperative., (2022).
Methods-only references
MeSH terms
Grants and funding
- UM1 HG008895/HG/NHGRI NIH HHS/United States
- R03NS108145/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 5U01HG009088/U.S. Department of Health & Human Services | NIH | National Human Genome Research Institute (NHGRI)
- R03 NS108145/NS/NINDS NIH HHS/United States
- R01 NS117544/NS/NINDS NIH HHS/United States

