A randomized controlled non-inferiority trial of placebo versus macrolide antibiotics for Mycoplasma pneumoniae infection in children with community-acquired pneumonia: trial protocol for the MYTHIC Study
- PMID: 39363201
- PMCID: PMC11450998
- DOI: 10.1186/s13063-024-08438-6
A randomized controlled non-inferiority trial of placebo versus macrolide antibiotics for Mycoplasma pneumoniae infection in children with community-acquired pneumonia: trial protocol for the MYTHIC Study
Abstract
Background: Mycoplasma pneumoniae is a major cause of community-acquired pneumonia (CAP) in school-aged children. Macrolides are the first-line treatment for this infection. However, it is unclear whether macrolides are effective in treating M. pneumoniae CAP, mainly due to limitations in microbiological diagnosis of previous studies. The extensive global use of macrolides has led to increasing antimicrobial resistance. The overall objective of this trial is to produce efficacy data for macrolide treatment in children with M. pneumoniae CAP.
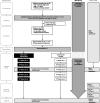
Methods: The MYTHIC Study is a randomized, double-blind, placebo-controlled, multicenter, non-inferiority trial in 13 Swiss pediatric centers. Previously healthy ambulatory and hospitalized children aged 3-17 years with clinically diagnosed CAP will be screened with a sensitive and commercially available M. pneumoniae-specific IgM lateral flow assay from capillary blood. Mycoplasma pneumoniae infection in screened patients will be verified retrospectively by respiratory PCR (reference test) and IgM antibody-secreting cell enzyme-linked immunospot (ELISpot) assay (confirmatory test for distinguishing between carriage and infection). Patients will be randomized 1:1 to receive a 5-day treatment of macrolides (azithromycin) or placebo. The co-primary endpoints are (1) time to normalization of all vital signs, including body temperature, respiratory rate, heart rate, and saturation of peripheral oxygen (efficacy), and (2) CAP-related change in patient care status (i.e., admission, re-admission, or intensive care unit transfer) within 28 days (safety). Secondary outcomes include adverse events (AEs), as well as antimicrobial and anti-inflammatory effects. For both co-primary endpoints, we aim to show non-inferiority of placebo compared to macrolide treatment. We expect no macrolide effect (hazard ratio of 1, absolute risk difference of 0) and set the corresponding non-inferiority margins to 0.7 and -7.5%. The "at least one" success criterion is used to handle multiplicity with the two co-primary endpoints. With a power of 80% to reject at least one null hypothesis at a one-sided significance level of 1.25%, 376 patients will be required.
Discussion: This trial will produce efficacy data for macrolide treatment in children with M. pneumoniae CAP that might help to reduce the prescription of antibiotics and therefore contribute to the global efforts toward reducing antimicrobial resistance.
Trial registration: ClinicalTrials.gov, NCT06325293. Registered on 24 April 2024.
Keywords: Anti-inflammatory; Antimicrobial; Atypical pneumonia; Azithromycin; Carriage; Colonization; Diagnosis; Resistance; Respiratory tract infection; Stewardship.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Meyer Sauteur PM, Beeton ML, on behalf of the ESGMAC and the ESGMAC Mycoplasma pneumoniae Surveillance (MAPS) study group. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. 2024;5:e100–1. - PubMed
-
- Meyer Sauteur PM, Beeton ML, on behalf of the ESGMAC and the ESGMAC Mycoplasma pneumoniae Surveillance (MAPS) study group. Pneumonia outbreaks due to re-emergence of Mycoplasma pneumoniae. Lancet Microbe. 2024;5:e514. - PubMed
-
- Gerber JS, Kronman MP, Ross RK, Hersh AL, Newland JG, Metjian TA, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34:1252–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

