Safety and pharmacokinetic properties of a new formulation of parenteral artesunate in healthy Thai volunteers
- PMID: 39363296
- PMCID: PMC11450984
- DOI: 10.1186/s12936-024-05085-9
Safety and pharmacokinetic properties of a new formulation of parenteral artesunate in healthy Thai volunteers
Abstract
Background: Parenteral artesunate is the first-line therapy for severe malaria. Artesunate, in its current formulation, must be prepared immediately before administration by first dissolving in sodium bicarbonate solution and then diluting in saline. A novel solvent for rapid and stable single step reconstitution of artesunate was recently developed showing improved solubility and stability. This study aimed to compare the safety and pharmacokinetic properties of the currently available and newly developed parenteral formulation of artesunate in healthy Thai volunteers.
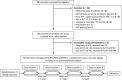
Methods: This was an open-label, randomized, 4 periods, 4-treatments, 24-sequence, single-dose, cross-over study in 72 male and female healthy Thai volunteers. Frequent pharmacokinetic samples were collected in all volunteers at each dose occasion. Observed concentration-time profiles were analysed with a non-compartmental approach followed by a bioequivalence evaluation.
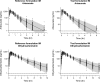
Results: Both intramuscular and intravenous administrations of the new parenteral formulation of artesunate were safe and well-tolerated, with no additional safety signals compared to the currently used formulation. The pharmacokinetic properties of artesunate and its active metabolite, dihydroartemisinin, were well-characterized, and showed rapid conversion of artesunate into dihydroartemisinin. Intramuscular administration of the newly formulated artesunate resulted in almost complete bioavailability of dihydroartemisinin. The pharmacokinetic properties were similar between the old and new formulation.
Conclusions: The new and more easily prepared formulation of artesunate was safe and well-tolerated, with similar pharmacokinetic properties compared to the currently used formulation. Dihydroartemisinin, the active metabolite responsible for the majority of the anti-malarial effect, showed equivalent exposure after both intravenous and intramuscular administration of artesunate, suggesting that both routes of administration should generate comparable therapeutic effects.
Trial registration: The study was registered to clinicaltrials.gov (#TCTR20170907002).
Keywords: Artesunate; Bioequivalence; Formulation; Healthy volunteer; Intramuscular; Intravenous; Malaria; Pharmacokinetics.
© 2024. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures


References
-
- WHO. World malaria report 2023: tracking progress and gaps in the global response to malaria. Geneva: World Health Organization; 2023. p. 2023.
-
- Piyaphanee W, Krudsood S, Tangpukdee N, Thanachartwet W, Silachamroon U, Phophak N, et al. Emergence and clearance of gametocytes in uncomplicated Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;74:432–5. - PubMed
-
- WHO. Guidelines for malaria. Geneva: World Health Organization; 2023.
-
- Dondorp A, Nosten F, Stepniewska K, Day N, White N. South East Asian Quinine Artesunate Malaria Trial Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

