Survey on adverse events associated with drug therapy for breast cancer patients
- PMID: 39363303
- PMCID: PMC11447999
- DOI: 10.1186/s12905-024-03355-x
Survey on adverse events associated with drug therapy for breast cancer patients
Abstract
Background: In the breast cancer treatment, there may be a gap between patients' information needs and physicians' perceptions. To address this issue, we conducted a comprehensive questionnaire survey aimed to assess the specific information needs of patients regarding the adverse events (AEs) associated with treatment.
Methods: A web-based questionnaire survey (UMIN000049280: Registered on October 31, 2022) was conducted in patients with a history of breast cancer treatment. Responses were obtained regarding AEs experienced, AEs for which remedies were identified, AEs patients sought to prevent, and pre-treatment information on AEs patients desired to have.
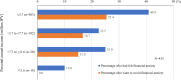
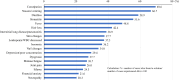
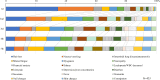
Results: Data from 435 breast cancer patients were analyzed. The most common AEs reported included hair loss (93.3%), malaise/fatigue (89.4%), nail changes (83.2%), dysgeusia (69.0%), leukopenia/white blood cell decreased (65.1%), neuropathy (62.3%), and nausea/vomiting (61.4%). Financial anxiety was reported in 35.2% of the participants. AEs for which a minority of patients found effective solutions included neuropathy (20.3%), financial anxiety (21.6%), edema (24.3%), joint pain (26.0%), and malaise/fatigue (26.7%). Patients expressed the greatest desire to avoid hair loss (34.7%), followed by nausea/vomiting (23.7%), interstitial lung disease/pneumonitis (5.5%), malaise/fatigue (5.1%), and dysgeusia (5.1%). The most commonly requested pre-treatment information regarding AEs was their duration, followed by prevention methods, management strategies, time to onset, and the impact on daily life.
Conclusions: This survey highlights the existence of significant unmet medical needs among breast cancer patients, due to the inadequate solutions available for managing AEs associated with various therapeutic agents. In addition, the survey revealed that patients have different information needs regarding different types of AEs.
Keywords: Adverse events; Breast cancer; PRO; Patients’ need; Questionnaire survey.
© 2024. The Author(s).
Conflict of interest statement
Fumikata Hara, Reiko Nagasaki, Naomi Sakurai, and Shinji Ohno received honorarium from Daiichi Sankyo Co., Ltd. Reiko Minami, Tadahiro Izutani, Takahiro Yoshida, and Ayako Arai are employees of Daiichi Sankyo Co., Ltd.
Figures


References
-
- Japanese Breast Cancer Society. Breast Cancer Clinical Practice Guideline. 2022. (in Japanese). Available from URL: https://jbcs.xsrv.jp/guideline/2022/ Accessed 11 December 2023.
-
- Barroso-Sousa R, Santana IA, Testa L, de Melo Gagliato D, Mano MS. Biological therapies in breast cancer: common toxicities and management strategies. Breast. 2013;22:1009–18. 10.1016/j.breast.2013.09.009. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

