Targeting the TDP-43 low complexity domain blocks spreading of pathology in a mouse model of ALS/FTD
- PMID: 39363348
- PMCID: PMC11448013
- DOI: 10.1186/s40478-024-01867-z
Targeting the TDP-43 low complexity domain blocks spreading of pathology in a mouse model of ALS/FTD
Abstract
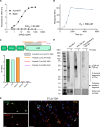
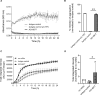
Abnormal cytoplasmic localization and accumulation of pathological transactive response DNA binding protein of 43 kDa (TDP-43) underlies several devastating diseases such as amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP). A key element is the correlation between disease progression and spatio-temporal propagation of TDP-43-mediated pathology in the central nervous system. Several lines of evidence support the concept of templated aggregation and cell to cell spreading of pathological TDP-43. To further investigate this mechanism in vivo, we explored the efficacy of capturing and masking the seeding-competent region of extracellular TDP-43 species. For this, we generated a novel monoclonal antibody (mAb), ACI-6677, that targets the pathogenic protease-resistant amyloid core of TDP-43. ACI-6677 has a picomolar binding affinity for TDP-43 and is capable of binding to all C-terminal TDP-43 fragments. In vitro, ACI-6677 inhibited TDP-43 aggregation and boosted removal of pathological TDP-43 aggregates by phagocytosis. When injecting FTLD-TDP brain extracts unilaterally in the CamKIIa-hTDP-43NLSm mouse model, ACI-6677 significantly limited the induction of phosphorylated TDP-43 (pTDP-43) inclusions. Strikingly, on the contralateral side, the mAb significantly prevented pTDP-43 inclusion appearance exemplifying blocking of the spreading process. Taken together, these data demonstrate for the first time that an immunotherapy targeting the protease-resistant amyloid core of TDP-43 has the potential to restrict spreading, substantially slowing or stopping progression of disease.
Keywords: ALS; FTD; Immunotherapy; Neuropathology; Pathomechanism; Spreading; TDP-43.
© 2024. The Author(s).
Conflict of interest statement
T.A. and T.S. are coinventors on a patent application, publication number WO2020/234473. R.O., T.A. and T.S. are coinventors on a patent application, publication number WO2022/034228. E.C., M.R., R.O., A.F., K.P., A.P., M.V., T.S. are employees of AC Immune and entitled to options and/or shares. M.A. and T.A. were employee of AC Immune at the time of this study. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

