Impact of a teat disinfectant based on Lactococcus cremoris on the cow milk proteome
- PMID: 39363353
- PMCID: PMC11448288
- DOI: 10.1186/s12917-024-04014-x
Impact of a teat disinfectant based on Lactococcus cremoris on the cow milk proteome
Abstract
Background: Dairy cow milking practices require cleaning and disinfection of the teat skin before and after milking to ensure the safety and quality of milk and prevent intramammary infections. Antimicrobial proteins of natural origin can be valuable alternatives to traditional disinfectants. In a recent field trial, we demonstrated that a teat dip based on a nisin A-producing Lactococcus cremoris (L) had comparable efficacy to conventional iodophor dip (C) in preventing dairy cow mastitis. Here, we present the differential shotgun proteomics investigation of the milk collected during the trial.
Methods: Four groups of quarter milk samples with low (LSCC) and high somatic cell count (HSCC) collected at the beginning (T0) and end (TF) of the trial were analyzed for a total of 28 LSCC (14 LSCC T0 and 14 LSCC TF) and 12 HSCC (6 HSCC T0 and 6 HSCC TF) samples. Milk proteins were digested into peptides, separated by nanoHPLC, and analyzed by tandem mass spectrometry (LC-MS/MS) on an Orbitrap Fusion Tribrid mass spectrometer. The proteins were identified with MaxQuant and interaction networks of the differential proteins were investigated with STRING. The proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD045030.
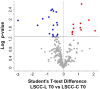
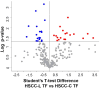
Results: In healthy milk (LSCC), we detected 90 and 80 differential proteins at T0 and TF, respectively. At TF, the Lactococcus group showed higher levels of antimicrobial proteins. In mastitis milk (HSCC), we detected 88 and 106 differential proteins at T0 and TF, respectively. In the Lactococcus group, 14 proteins with antimicrobial and immune defense functions were enriched at TF vs. 4 proteins at T0. Cathelicidins were among the most relevant enriched proteins. Western immunoblotting validation confirmed the differential abundance.
Conclusions: At T0, the proteomic differences observed in healthy milk of the two groups were most likely dependent on physiological variation. On the other hand, antimicrobial and immune defense functions were higher in the milk of cows with mammary gland inflammation of the Lactococcus-treated group. Among other factors, the immunostimulatory action of nisin A might be considered as a contributor.
Keywords: Antimicrobial; Cathelicidin; Immunostimulation; Milk proteomics; Milking routine; Shotgun proteomics; Teat dipping.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Dore S, Ferrini AM, Appicciafuoco B, Massaro MR, Sotgiu G, Liciardi M, et al. Efficacy of a terpinen-4-ol based dipping for post-milking teat disinfection in the prevention of mastitis in dairy sheep. J Essent Oil Res. 2019;31:19–26.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

