Targeted Therapy of Osteoarthritis via Intra-Articular Delivery of Lipid-Nanoparticle-Encapsulated Recombinant Human FGF18 mRNA
- PMID: 39363784
- PMCID: PMC11582510
- DOI: 10.1002/adhm.202400804
Targeted Therapy of Osteoarthritis via Intra-Articular Delivery of Lipid-Nanoparticle-Encapsulated Recombinant Human FGF18 mRNA
Abstract
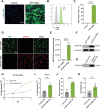
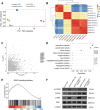
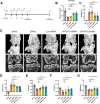
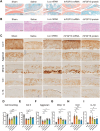
Fibroblast growth factor 18 (FGF18) emerges as a promising therapeutic target for osteoarthritis (OA). In this study, a novel articular cavity-localized lipid nanoparticle (LNP) named WG-PL14 is developed. This optimized formulation has a nearly 30-fold increase in mRNA expression as well as better articular cavity enrichment compared to commercial lipids MC3 when performing intra-articular injection. Then, a mRNA sequence encoding recombinant human FGF18 (rhFGF18) for potential mRNA therapy in OA is optimized. In vitro assays confirm the translation of rhFGF18 mRNA into functional proteins within rat and human chondrocytes, promoting cell proliferation and extracellular matrix (ECM) synthesis. Subsequently, the therapeutic efficacy of the LNP-rhFGF18 mRNA complex is systematically assessed in a mouse OA model. The administration exhibits several positive outcomes, including an improved pain response, upregulation of ECM-related genes (e.g., AGRN and HAS2), and remodeling of subchondral bone homeostasis compared to a control group. Taken together, these findings underscore the potential of localized LNP-rhFGF18 mRNA therapy in promoting the regeneration of cartilage tissue and mitigating the progression of OA.
Keywords: lipid nanoparticles; mRNA; osteoarthritis; recombinant human FGF18.
© 2024 The Author(s). Advanced Healthcare Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hunter D. J., Bierma‐Zeinstra S., Lancet 2019, 393, 1745. - PubMed
-
- Safiri S., Kolahi A.‐A., Smith E., Hill C., Bettampadi D., Mansournia M. A., Hoy D., Ashrafi‐Asgarabad A., Sepidarkish M., Almasi‐Hashiani A., Collins G., Kaufman J., Qorbani M., Moradi‐Lakeh M., Woolf A. D., Guillemin F., March L., Cross M., Ann. Rheum. Dis. 2020, 79, 819. - PubMed
-
- Latourte A., Kloppenburg M., Richette P., Nat Rev Rheumatol 2020, 16, 673. - PubMed
MeSH terms
Substances
Grants and funding
- U23A6009/the Regional Innovation Joint Fund of the National Natural Science Foundation of China (Integrated Project)
- PKU2023LCXQ007/Clinical Medicine Plus X-Young Scholars Project of Peking University
- Z22022/Natural Science Foundation of Beijing Municipality
- JQ23029/Natural Science Foundation of Beijing Municipality
- L234024/Natural Science Foundation of Beijing Municipality
- U23A6009/National Natural Science Foundation of China
- HY2021-8/National Natural Science Foundation of China
- 82373807/National Natural Science Foundation of China
- 20220484100/Beijing Nova Program
- 20230484448/Beijing Nova Program
- Emerging Engineering Interdisciplinary-Young Scholars Project, Peking University
- Z231100007223001/Beijing Municipal Science and Technology Commission, Adminitrative Commission of Zhongguancun Science Park
- Z231100007223012/Beijing Municipal Science and Technology Commission, Adminitrative Commission of Zhongguancun Science Park
- PKU2023XGK011/Fundamental Research Funds for the Central Universities
- 2023YFC3405000/Key Technologies Research and Development Program
LinkOut - more resources
Full Text Sources
Medical

