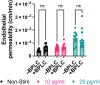Pericytes Enrich the Basement Membrane and Reduce Neutrophil Transmigration in an In Vitro Model of Peripheral Inflammation at the Blood-Brain Barrier
- PMID: 39363889
- PMCID: PMC11447289
- DOI: 10.34133/bmr.0081
Pericytes Enrich the Basement Membrane and Reduce Neutrophil Transmigration in an In Vitro Model of Peripheral Inflammation at the Blood-Brain Barrier
Abstract
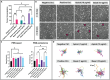
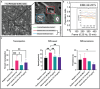
Sepsis is the most lethal and expensive condition treated in intensive care units. Sepsis survivors frequently suffer long-term cognitive impairment, which has been linked to the breakdown of the blood-brain barrier (BBB) during a sepsis-associated "cytokine storm". Because animal models poorly recapitulate sepsis pathophysiology, human models are needed to understand sepsis-associated brain injury and to develop novel therapeutic strategies. With the concurrent emergence of tissue chip technologies and the maturation of protocols for human induced pluripotent stem cell (hiPSC), we can now develop advanced in vitro models of the human BBB and immune system to understand the relationship between systemic inflammation and brain injury. Here, we present a BBB model of the primary barrier developed on the μSiM (microphysiological system enabled by an ultrathin silicon nanomembrane) tissue chip platform. The model features isogenically matched hiPSC-derived extended endothelial culture method brain microvascular endothelial cell-like cells (EECM-BMEC-like cells) and brain pericyte-like cells (BPLCs) in a back-to-back coculture separated by the ultrathin (100 nm) membrane. Both endothelial monocultures and cocultures with pericytes responded to sepsis-like stimuli, with increased small-molecule permeability, although no differences were detected between culture conditions. Conversely, BPLC coculture reduced the number of neutrophils that crossed the EECM-BMEC-like cell monolayer under sepsis-like stimulation. Interestingly, this barrier protection was not seen when the stimulus originated from the tissue side. Our studies are consistent with the reported role for pericytes in regulating leukocyte trafficking during sepsis but indicate that EECM-BMEC-like cells alone are sufficient to maintain the restrictive small-molecule permeability of the BBB.
Copyright © 2024 Molly C. McCloskey et al.
Conflict of interest statement
Competing interests: J.L.M. and T.R.G. are cofounders of SiMPore and hold equity interests in the company. SiMPore is commercializing ultrathin silicon-based technologies including the membranes used in this study. B.D.G., E.V.S., S.P.P., and B.E. are inventors on patent application PCT/US2021/052421 related to EECM-BMEC-like cells. S.P.P. and E.V.S. are inventors on patent US11643636B2 related to BPLCs.
Figures