Synergistic Effects of Silica-Supported Iron-Cobalt Catalysts for CO2 Reduction to Prebiotic Organics
- PMID: 39363906
- PMCID: PMC7616659
- DOI: 10.1002/cctc.202301218
Synergistic Effects of Silica-Supported Iron-Cobalt Catalysts for CO2 Reduction to Prebiotic Organics
Abstract
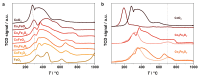
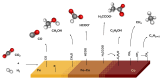
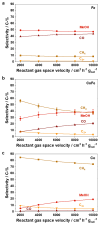
To test the ability of geochemical surfaces in serpentinizing hydrothermal systems to catalyze reactions from which metabolism arose, we investigated H2-dependent CO2 reduction toward metabolic intermediates over silica-supported Co-Fe catalysts. Supported catalysts converted CO2 to various products at 180 °C and 2.0 MPa. The liquid product phase included formate, acetate, and ethanol, while the gaseous product phase consisted of CH4, CO, methanol, and C2-C7 linear hydrocarbons. The 1/1 ratio CoFe alloy with the same composition as the natural mineral wairauite yielded the highest concentrations of formate (6.0 mM) and acetate (0.8 mM), which are key intermediates in the acetyl-coenzyme A (acetyl-CoA) pathway of CO2 fixation. While Co-rich catalysts were proficient at hydrogenation, yielding mostly CH4, Fe-rich catalysts favored the formation of CO and methanol. Mechanistic studies indicated intermediate hydrogenation and C-C coupling activities of alloyed CoFe, in contrast to physical mixtures of both metals. Co in the active site of Co-Fe catalysts performed a similar reaction as tetrapyrrole-coordinated Co in the corrinoid iron-sulfur (CoFeS) methyl transferase in the acetyl-CoA pathway. In a temperature range characteristic for deeper regions of serpentinizing systems, oxygenate product formation was favored at lower, more biocompatible temperatures.
Keywords: CO2 Fixation; CoFe Alloy; Heterogeneous catalysis; Hydrothermal Vent; Prebiotic Chemistry.
Conflict of interest statement
Conflict of Interests The authors declare no conflict of interest.
Figures





References
-
- Oparin AI. The origin of life. The MacMillan Company; New York City: 1938. p. 127.
-
- Haldane JBS. Rationalist Annual. 1929;148:3–10.
-
- Chyba C. Nature. 1992;355:125–132. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
