Glucose metabolism impairment as a hallmark of progressive myoclonus epilepsies: a focus on neuronal ceroid lipofuscinoses
- PMID: 39364042
- PMCID: PMC11447523
- DOI: 10.3389/fncel.2024.1445003
Glucose metabolism impairment as a hallmark of progressive myoclonus epilepsies: a focus on neuronal ceroid lipofuscinoses
Abstract
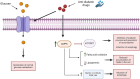
Glucose is the brain's main fuel source, used in both energy and molecular production. Impaired glucose metabolism is associated with adult and pediatric neurodegenerative diseases such as Alzheimer's disease (AD), Parkinson's disease (PD), GLUT1 deficiency syndrome, and progressive myoclonus epilepsies (PMEs). PMEs, a group of neurological disorders typical of childhood and adolescence, account for 1% of all epileptic diseases in this population worldwide. Diffuse glucose hypometabolism is observed in the brains of patients affected by PMEs such as Lafora disease (LD), dentatorubral-pallidoluysian (DRPLA) atrophy, Unverricht-Lundborg disease (ULD), and myoclonus epilepsy with ragged red fibers (MERRFs). PMEs also include neuronal ceroid lipofuscinoses (NCLs), a subgroup in which lysosomal and autophagy dysfunction leads to progressive loss of vision, brain atrophy, and cognitive decline. We examine the role of impaired glucose metabolism in neurodegenerative diseases, particularly in the NCLs. Our literature review, which includes findings from case reports and animal studies, reveals that glucose hypometabolism is still poorly characterized both in vitro and in vivo in the different NCLs. Better identification of the glucose metabolism pathway impaired in the NCLs may open new avenues for evaluating the therapeutic potential of anti-diabetic agents in this population and thus raise the prospect of a therapeutic approach able to delay or even halt disease progression.
Keywords: anti-diabetics; glucose metabolism; neurodegeneration; neuronal ceroid lipofuscinosis; progressive myoclonic epilepsies.
Copyright © 2024 Santucci, Bernardi, Vivarelli, Santorelli and Marchese.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Apelt J., Mehlhorn G., Schliebs R. (1999). Insulin-sensitive GLUT4 glucose transporters are Colocalized with GLUT3-expressing cells and demonstrate a chemically distinct neuron-specific localization in rat brain. J. Neurosci. Res. 57, 693–705. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

