Guiding the starting dose of the once-daily formulation of tacrolimus in " de novo" adult renal transplant patients: a population approach
- PMID: 39364055
- PMCID: PMC11447946
- DOI: 10.3389/fphar.2024.1456565
Guiding the starting dose of the once-daily formulation of tacrolimus in " de novo" adult renal transplant patients: a population approach
Abstract
Aims: The once-daily extended-release tacrolimus formulation (ER-Tac) has demonstrated similar efficacy and safety to the twice-daily immediate-release formulation (IR-Tac), but few population-based pharmacokinetic models have been developed in de novo kidney transplant patients to optimize doses. Therefore, this study aimed i) at developing a population pharmacokinetic model for ER-Tac in de novo adult kidney transplant patients ii) and identifying genetic factors and time-varying covariates predictive of pharmacokinetic variability to guide tacrolimus dosage during the early post-transplant period.
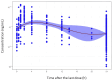
Methods: A total of 1,067 blood tacrolimus concentrations from 138 kidney transplant patients were analyzed. A total of 29 out of 138 patients were intensively sampled for 24 h on the day 5 post-transplantation; meanwhile, for the remaining patients, concentrations were collected on days 5, 10, and 15 after transplantation. Tacrolimus daily doses and genetic and demographic characteristics were retrieved from the medical files. Biochemistry time-varying covariates were obtained on different days over the pharmacokinetic (PK) study. A simultaneous PK analysis of all concentrations was carried out using the non-linear mixed-effects approach with NONMEM 7.5.
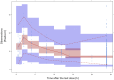
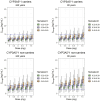
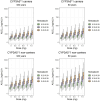
Results: A two-compartment model with linear elimination and delayed absorption best described the tacrolimus pharmacokinetics. Between-patient variability was associated with oral blood clearance (CL/F) and the central compartment distribution volume (Vc/F). Tacrolimus concentrations standardized to a hematocrit value of 45% significantly improved the model (p < 0.001). This method outperformed the standard covariate modeling of the hematocrit-blood clearance relationship. The effect of the CYP3A5 genotype was statistically (p < 0.001) and clinically significant on CL/F. The CL/F of patients who were CYP3A5*1 carriers was 51% higher than that of CYP3A5*1 non-carriers. Age also influenced CL/F variability (p < 0.001). Specifically, CL/F declined by 0.0562 units per each increased year from the value estimated in patients who were 60 years and younger.
Conclusion: The 36% between-patient variability in CL/F was explained by CYP3A5 genotype, age, and hematocrit. Hematocrit standardization to 45% explained the variability of tacrolimus whole-blood concentrations, and this was of utmost importance in order to better interpret whole-blood tacrolimus concentrations during therapeutic drug monitoring. The dose requirements of CYP3A5*/1 carriers in patients aged 60 years or younger would be highest, while CYP3A5*/1 non-carriers older than 60 years would require the lowest doses.
Keywords: CYP3A4; CYP3A5; ER-Tac; age; de novo-kidney transplant patients; hematocrit; population pharmacokinetics.
Copyright © 2024 Fernández-Alarcón, Nolberger, Vidal-Alabró, Rigo-Bonnin, Grinyó, Melilli, Montero, Manonelles, Coloma, Favà, Codina, Cruzado, Colom and Lloberas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Andrews L. M., Hesselink D. A., van Gelder T., Koch B. C. P., Cornelissen E. A. M., Brüggemann R. J. M., et al. (2018). A population pharmacokinetic model to predict the individual starting dose of tacrolimus following pediatric renal transplantation. Clin. Pharmacokinet. 57 (4), 475–489. 10.1007/s40262-017-0567-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

