Isoniazid-historical development, metabolism associated toxicity and a perspective on its pharmacological improvement
- PMID: 39364056
- PMCID: PMC11447295
- DOI: 10.3389/fphar.2024.1441147
Isoniazid-historical development, metabolism associated toxicity and a perspective on its pharmacological improvement
Abstract
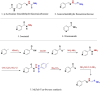
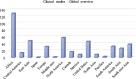
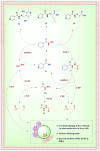
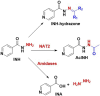
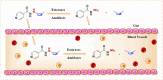
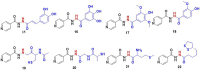
Despite the extraordinary anti-tubercular activity of isoniazid (INH), the drug-induced hepatotoxicity and peripheral neuropathy pose a significant challenge to its wider clinical use. The primary cause of INH-induced hepatotoxicity is in vivo metabolism involving biotransformation on its terminal -NH2 group owing to its high nucleophilic nature. The human N-acetyltransferase-2 enzyme (NAT-2) exploits the reactivity of INH's terminal -NH2 functional group and inactivates it by transferring the acetyl group, which subsequently converts to toxic metabolites. This -NH2 group also tends to react with vital endogenous molecules such as pyridoxine, leading to their deficiency, a major cause of peripheral neuropathy. The elevation of liver functional markers is observed in 10%-20% of subjects on INH treatment. INH-induced risk of fatal hepatitis is about 0.05%-1%. The incidence of peripheral neuropathy is 2%-6.5%. In this review, we discuss the genesis and historical development of INH, and different reported mechanisms of action of INH. This is followed by a brief review of various clinical trials in chronological order, highlighting treatment-associated adverse events and their occurrence rates, including details such as geographical location, number of subjects, dosing concentration, and regimen used in these clinical studies. Further, we elaborated on various known metabolic transformations highlighting the involvement of the terminal -NH2 group of INH and corresponding host enzymes, the structure of different metabolites/conjugates, and their association with hepatotoxicity or neuritis. Post this deliberation, we propose a hydrolysable chemical derivatives-based approach as a way forward to restrict this metabolism.
Keywords: N-acetyltransferase; hepatotoxicity; isoniazid; metabolism; peripheral neuropathy; prodrug.
Copyright © 2024 Sankar, Chauhan, Singh and Mahajan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agarwal A., Bansal R., Sharma S., Meena M., Airun M. (2016). Near fatal poisoning by isoniazid and rifampicin-a case report and review of literature. Indian J. Forensic Med. Toxicol. 10 (1), 147–150. 10.5958/0973-9130.2016.00034.7 - DOI
Publication types
LinkOut - more resources
Full Text Sources

