A virulence-associated small RNA MTS1338 activates an ABC transporter CydC for rifampicin efflux in Mycobacterium tuberculosis
- PMID: 39364170
- PMCID: PMC11446857
- DOI: 10.3389/fmicb.2024.1469280
A virulence-associated small RNA MTS1338 activates an ABC transporter CydC for rifampicin efflux in Mycobacterium tuberculosis
Abstract
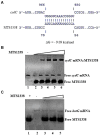
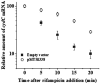
The efficacy of the tuberculosis treatment is restricted by innate drug resistance of Mycobacterial tuberculosis and its ability to acquire resistance to all anti-tuberculosis drugs in clinical use. A profound understanding of bacterial ploys that decrease the effectiveness of drugs would identify new mechanisms for drug resistance, which would subsequently lead to the development of more potent TB therapies. In the current study, we identified a virulence-associated small RNA (sRNA) MTS1338-driven drug efflux mechanism in M. tuberculosis. The treatment of a frontline antitubercular drug rifampicin upregulated MTS1338 by >4-fold. Higher intrabacterial abundance of MTS1338 increased the growth rate of cells in rifampicin-treated conditions. This fact was attributed by the upregulation of an efflux protein CydC by MTS1338. Gel-shift assay identified a stable interaction of MTS1338 with the coding region of cydC mRNA thereby potentially stabilizing it at the posttranscriptional level. The drug efflux measurement assays revealed that cells with higher MTS1338 abundance accumulate less drug in the cells. This study identified a new regulatory mechanism of drug efflux controlled by an infection-induced sRNA in M. tuberculosis.
Keywords: antimicrobial resistance; drug efflux; efflux protein; gene regulation; small RNAs.
Copyright © 2024 Singh and Dutta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Small RNA MTS1338 Configures a Stress Resistance Signature in Mycobacterium tuberculosis.Int J Mol Sci. 2023 Apr 27;24(9):7928. doi: 10.3390/ijms24097928. Int J Mol Sci. 2023. PMID: 37175635 Free PMC article.
-
Mycobacterium tuberculosis Small RNA MTS1338 Confers Pathogenic Properties to Non-Pathogenic Mycobacterium smegmatis.Microorganisms. 2021 Feb 17;9(2):414. doi: 10.3390/microorganisms9020414. Microorganisms. 2021. PMID: 33671144 Free PMC article.
-
Transcriptional activation of the Mycobacterium tuberculosis virulence-associated small RNA MTS1338 by the response regulators DosR and PhoP.FEBS Lett. 2024 May;598(9):1034-1044. doi: 10.1002/1873-3468.14882. Epub 2024 Apr 19. FEBS Lett. 2024. PMID: 38639734
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
Efflux pump inhibitors as a promising adjunct therapy against drug resistant tuberculosis: a new strategy to revisit mycobacterial targets and repurpose old drugs.Expert Rev Anti Infect Ther. 2020 Aug;18(8):741-757. doi: 10.1080/14787210.2020.1760845. Epub 2020 May 20. Expert Rev Anti Infect Ther. 2020. PMID: 32434397 Review.
Cited by
-
Based on quorum sensing: reverse effect of traditional Chinese medicine on bacterial drug resistance mechanism.Front Cell Infect Microbiol. 2025 Jun 3;15:1582003. doi: 10.3389/fcimb.2025.1582003. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40568697 Free PMC article. Review.
References
-
- Acuña L. G., Barros M. J., Peñaloza D., Rodas P. I., Paredes-Sabja D., Fuentes J. A., et al. . (2016). A feed-forward loop between SroC and MgrR small RNAs modulates the expression of eptB and susceptibility of polymyxin B in Salmonella Typhimurium. Microbiology 162, 1996–2004. doi: 10.1099/mic.0.000365, PMID: - DOI - PubMed
-
- Arnvig K. B., Comas I., Thomson N. R., Houghton J., Boshoff H. I., Croucher N. J., et al. . (2011). Sequence-based analysis uncovers an abundance of noncoding RNA in the total transcriptome of Mycobacterium tuberculosis. PLoS Pathog. 7:e1002342. doi: 10.1371/journal.ppat.1002342, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

