Inhaled Corticosteroids Particle Size and Risk of Hospitalization Due to Exacerbations and All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. A Nationwide Cohort Study
- PMID: 39364225
- PMCID: PMC11448463
- DOI: 10.2147/COPD.S453524
Inhaled Corticosteroids Particle Size and Risk of Hospitalization Due to Exacerbations and All-Cause Mortality in Patients with Chronic Obstructive Pulmonary Disease. A Nationwide Cohort Study
Abstract
Background: Extra-fine particle inhaled corticosteroids (ICS) improve peripheral airway distribution, but their effect on risk of exacerbations and all-cause mortality in patients with chronic obstructive pulmonary disease (COPD) is unclear.
Methods: This observational cohort study compares patients with COPD who received extra-fine particle ICS to those who received standard particle size ICS from 2010 to 2017 while followed in outpatient clinics. The primary outcome was the time to a COPD exacerbation that required hospitalization, with all-cause mortality as a secondary outcome. Data were analyzed using an adjusted Cox proportional hazards model and a competing risk analysis. Two predefined subgroup analyses of patients treated with pressurised metered dose inhalers (pMDIs) and patients with a previous exacerbation history, was carried out. Lastly, we created a propensity score matched cohort as a sensitivity analysis.
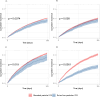
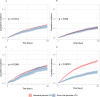
Results: Of the 40,489 patients included, 38,802 (95.8%) received stand particle size ICS and 1,687 (4.2%) received extra-fine particle ICS. In total 7,058 were hospitalized with a COPD exacerbation, and 4,346 died. No significant protective effect of extra-fine particle ICS against hospitalization due to COPD exacerbations (HR 0.93, 95% CI 0.82-1.05, p=0.23) or all-cause mortality (HR 1.00, 95% CI 0.85-1.17, p=0.99) was found when compared to standard particle size ICS. However, in the subgroup analysis of patients treated with pMDIs, extra-fine particle ICS was associated with reduction in risk of exacerbations (HR 0.72, 95% CI 0.63-0.82, p<0.001) and all-cause mortality (HR 0.72, 95% CI 0.61-0.86, p<0.001).
Conclusion: The administration of extra-fine particle ICS was not associated with reduced risk of exacerbations or all-cause mortality in our primary analysis. A subgroup consisting of patients treated with pMDIs suggested potential protective benefits.
Keywords: COPD; COPD exacerbations; Inhaled Corticosteroids; Particle size.
© 2024 Heerfordt et al.
Conflict of interest statement
ZBH received grants from the Independent Research Fund Denmark, the Lundbeck Foundation, and the Danish Cancer Society. CH received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events and support for attending meetings and/or travel from Boehringer-Ingelheim. AF received payment for presentations at educational events from AstraZeneca, GSK, and Chiesi, and received support for attending ERS 2023 by AstraZeneca. AGM received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from GlaxoSmithKline. TBS received consulting fees from GSK, Novo Nordisk, Amgen, CSL Seqirus, Novartis, Boston Scientific, GE Healthcare, IQVIA, Parexel, and Sanofi Pasteur, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer, Sanofi Pasteur, and GSK, support for attending meetings and/or travel from AstraZeneca, and is part of the Data Safety Monitoring Board or Advisory Board for GSK and Sanofi Pasteur, and received equipment for his department from GE. The other authors declare no conflicts of interest in this work.
Figures

References
-
- Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52(6). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

