Next-Generation Therapies for Multiple Myeloma
- PMID: 39364307
- PMCID: PMC11449476
- DOI: 10.1146/annurev-cancerbio-061421-014236
Next-Generation Therapies for Multiple Myeloma
Abstract
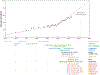
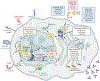
Recent therapeutic advances have significantly improved the outcome for patients with multiple myeloma (MM). The backbone of successful standard therapy is the combination of Ikaros degraders, glucocorticoids, and proteasome inhibitors that interfere with the integrity of myeloma-specific superenhancers by directly or indirectly targeting enhancer-bound transcription factors and coactivators that control expression of MM dependency genes. T cell engagers and chimeric antigen receptor T cells redirect patients' own T cells onto defined tumor antigens to kill MM cells. They have induced complete remissions even in end-stage patients. Unfortunately, responses to both conventional therapy and immunotherapy are not durable, and tumor heterogeneity, antigen loss, and lack of T cell fitness lead to therapy resistance and relapse. Novel approaches are under development to target myeloma-specific vulnerabilities, as is the design of multimodality immunological approaches, including and beyond T cells, that simultaneously recognize multiple epitopes to prevent antigen escape and tumor relapse.
Keywords: CAR T cell; EP300; T cell engagers; bispecific antibody; chimeric antigen receptor; multiple myeloma; superenhancers.
Figures

References
-
- Abdallah A-O, Cowan AJ, Leleu X, Touzeau C, Lipe B, et al. 2022. Updated interim results from a phase 1 study of HPN217, a half-life extended tri-specific T cell activating construct (TriTAC®) targeting B cell maturation antigen (BCMA) for relapsed/refractory multiple myeloma (RRMM). Blood 140(Suppl. 1):7284–85
-
- Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, et al. 2016. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol 3(4):e196–204 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
