Preoperative prediction of lymph node metastasis in endometrial cancer patients via an intratumoral and peritumoral multiparameter MRI radiomics nomogram
- PMID: 39364314
- PMCID: PMC11446724
- DOI: 10.3389/fonc.2024.1472892
Preoperative prediction of lymph node metastasis in endometrial cancer patients via an intratumoral and peritumoral multiparameter MRI radiomics nomogram
Abstract
Introduction: While lymph node metastasis (LNM) plays a critical role in determining treatment options for endometrial cancer (EC) patients, the existing preoperative methods for evaluating the lymph node state are not always satisfactory. This study aimed to develop and validate a nomogram based on intra- and peritumoral radiomics features and multiparameter magnetic resonance imaging (MRI) features to preoperatively predict LNM in EC patients.
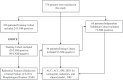
Methods: Three hundred and seventy-four women with histologically confirmed EC were divided into training (n = 220), test (n = 94), and independent validation (n = 60) cohorts. Radiomic features were extracted from intra- and peritumoral regions via axial T2-weighted imaging (T2WI) and apparent diffusion coefficient (ADC) mapping. A radiomics model (annotated as the Radscore) was established using the selected features from different regions. The clinical parameters were statistically analyzed. A nomogram was developed by combining the Radscore and the most predictive clinical parameters. Decision curve analysis (DCA) and the net reclassification index (NRI) were used to assess the clinical benefit of using the nomogram.
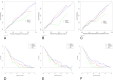
Results: Nine radiomics features were ultimately selected from the intra- and peritumoral regions via ADC mapping and T2WI. The nomogram combining the Radscore, serum CA125 level, and tumor area ratio achieved the highest AUCs in the training, test and independent validation sets (nomogram vs. Radscore vs. clinical model: 0.878 vs. 0.850 vs. 0.674 (training), 0.877 vs. 0.838 vs. 0.668 (test), and 0.864 vs. 0.836 vs. 0.618 (independent validation)). The DCA and NRI results revealed the nomogram had greater diagnostic performance and net clinical benefits than the Radscore alone.
Conclusion: The combined intra- and peritumoral region multiparameter MRI radiomics nomogram showed good diagnostic performance and could be used to preoperatively predict LNM in patients with EC.
Keywords: endometrial cancer; lymph node; lymphatic metastasis; magnetic resonance imaging; radiomics.
Copyright © 2024 Yan, Zhao, Deng and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
MRI-based multiregional radiomics for predicting lymph nodes status and prognosis in patients with resectable rectal cancer.Front Oncol. 2023 Jan 4;12:1087882. doi: 10.3389/fonc.2022.1087882. eCollection 2022. Front Oncol. 2023. PMID: 36686763 Free PMC article.
-
Preoperative prediction of lymphovascular space invasion in endometrioid adenocarcinoma: an MRI-based radiomics nomogram with consideration of the peritumoral region.Acta Radiol. 2023 Sep;64(9):2636-2645. doi: 10.1177/02841851231181681. Epub 2023 Jun 13. Acta Radiol. 2023. PMID: 37312525
-
MRI-based multiregional radiomics for preoperative prediction of tumor deposit and prognosis in resectable rectal cancer: a bicenter study.Eur Radiol. 2023 Nov;33(11):7561-7572. doi: 10.1007/s00330-023-09723-9. Epub 2023 May 9. Eur Radiol. 2023. PMID: 37160427
-
Research progress in multimodal radiomics of rectal cancer tumors and peritumoral regions in MRI.Abdom Radiol (NY). 2025 May 31. doi: 10.1007/s00261-025-04965-1. Online ahead of print. Abdom Radiol (NY). 2025. PMID: 40448847 Review.
-
Advances in Radiomics Research for Endometrial Cancer: A Comprehensive Review.J Cancer. 2023 Oct 24;14(18):3523-3531. doi: 10.7150/jca.89347. eCollection 2023. J Cancer. 2023. PMID: 38021155 Free PMC article. Review.
Cited by
-
Engrailed-2 (EN2) protein in cervical mucus: a novel biomarker for endometrial carcinoma.Clin Transl Oncol. 2025 Jun;27(6):2484-2493. doi: 10.1007/s12094-024-03799-5. Epub 2024 Nov 28. Clin Transl Oncol. 2025. PMID: 39607581 Free PMC article.
-
Predictive value of models based on MRI radiomics and clinical indicators for lymphovascular space invasion in endometrial cancer.BMC Cancer. 2025 Apr 28;25(1):796. doi: 10.1186/s12885-025-14217-6. BMC Cancer. 2025. PMID: 40295942 Free PMC article.
-
Predicting recurrence risk in endometrial cancer: a multisequence MRI intratumoral and peritumoral radiomics nomogram approach.Front Oncol. 2025 May 6;15:1569729. doi: 10.3389/fonc.2025.1569729. eCollection 2025. Front Oncol. 2025. PMID: 40395329 Free PMC article.
-
Preoperative inflammatory markers and tumor markers in predicting lymphatic metastasis and postoperative complications in colorectal cancer: a retrospective study.BMC Surg. 2025 Feb 18;25(1):71. doi: 10.1186/s12893-025-02795-y. BMC Surg. 2025. PMID: 39966788 Free PMC article.
References
-
- Korkmaz V, Meydanli MM, Yalçın I, Sarl ME, Sahin H, Kocaman E, et al. . Comparison of three different risk-stratification models for predicting lymph node involvement in endometrioid endometrial cancer clinically confined to the uterus. J Gynecol Oncol. (2017) 28:e78. doi: 10.3802/jgo.2017.28.e78 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

