Humoral and cellular immune responses in vaccinated and unvaccinated children following SARS-CoV-2 Omicron infection
- PMID: 39364394
- PMCID: PMC11447454
- DOI: 10.1002/cti2.70008
Humoral and cellular immune responses in vaccinated and unvaccinated children following SARS-CoV-2 Omicron infection
Abstract
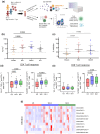
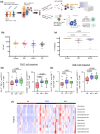
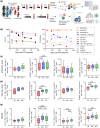
Objectives: The immune response in children elicited by SARS-CoV-2 Omicron infection alone or in combination with COVID-19 vaccination (hybrid immunity) is poorly understood. We examined the humoral and cellular immune response following SARS-CoV-2 Omicron infection in unvaccinated children and children who were previously vaccinated with COVID-19 mRNA vaccine.
Methods: Participants were recruited as part of a household cohort study conducted during the Omicron predominant wave (Jan to July 2022) in Victoria, Australia. Blood samples were collected at 1, 3, 6 and 12 months following COVID-19 diagnosis. Humoral immune responses to SARS-CoV-2 Spike proteins from Wuhan, Omicron BA.1, BA.4/5 and JN.1, as well as cellular immune responses to Wuhan and BA.1 were assessed.
Results: A total of 43 children and 113 samples were included in the analysis. Following Omicron infection, unvaccinated children generated low antibody responses but elicited Spike-specific CD4 and CD8 T-cell responses. In contrast, vaccinated children infected with the Omicron variant mounted robust humoral and cellular immune responses to both ancestral strain and Omicron subvariants. Hybrid immunity persisted for at least 6 months post infection, with cellular immune memory characterised by the generation of Spike-specific polyfunctional CD8 T-cell responses.
Conclusion: SARS-CoV-2 hybrid immunity in children is characterised by persisting SARS-CoV-2 antibodies and robust CD4 and CD8 T-cell activation and polyfunctional responses. Our findings contribute to understanding hybrid immunity in children and may have implications regarding COVID-19 vaccination and SARS-CoV-2 re-infections.
Keywords: SARS‐CoV‐2; cellular immune responses; children; hybrid immunity.
© 2024 The Author(s). Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
NWC received funding from the National Institute of Health for influenza and COVID‐19 research. All other authors declare no conflict of interest.
Figures

Similar articles
-
Persistent T cell-mediated immune responses against Omicron variants after the third COVID-19 mRNA vaccine dose.Front Immunol. 2023 Jan 23;14:1099246. doi: 10.3389/fimmu.2023.1099246. eCollection 2023. Front Immunol. 2023. PMID: 36756112 Free PMC article.
-
SARS-CoV-2 Omicron infection augments the magnitude and durability of systemic and mucosal immunity in triple-dose CoronaVac recipients.mBio. 2024 Apr 10;15(4):e0240723. doi: 10.1128/mbio.02407-23. Epub 2024 Mar 8. mBio. 2024. PMID: 38456703 Free PMC article.
-
Omicron BA.1-specific T-cell responses in adults vaccinated with CoronaVac or BNT162b2 in Hong Kong: an observational cohort study.Lancet Microbe. 2023 Jun;4(6):e418-e430. doi: 10.1016/S2666-5247(23)00006-X. Epub 2023 Apr 20. Lancet Microbe. 2023. PMID: 37086735 Free PMC article.
-
The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages.Virol Sin. 2022 Dec;37(6):786-795. doi: 10.1016/j.virs.2022.11.007. Epub 2022 Nov 23. Virol Sin. 2022. PMID: 36427646 Free PMC article. Review.
-
Humoral and Cellular Immune Responses of COVID-19 vaccines against SARS-Cov-2 Omicron variant: a systemic review.Int J Biol Sci. 2022 Jul 11;18(12):4629-4641. doi: 10.7150/ijbs.73583. eCollection 2022. Int J Biol Sci. 2022. PMID: 35874952 Free PMC article.
Cited by
-
Evaluation of Cellular Immune Responses After mRNA-1273 Vaccination in Children 6 Months to 11 Years of Age.J Infect Dis. 2025 Jun 2;231(5):e945-e955. doi: 10.1093/infdis/jiaf144. J Infect Dis. 2025. PMID: 40119775 Free PMC article. Clinical Trial.
-
Immunogenicity of the CoronaVac vaccine in children: a real-world study.Front Immunol. 2024 Dec 23;15:1504935. doi: 10.3389/fimmu.2024.1504935. eCollection 2024. Front Immunol. 2024. PMID: 39763646 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
