Recognizing Leber's Hereditary Optic Neuropathy to avoid delayed diagnosis and misdiagnosis
- PMID: 39364415
- PMCID: PMC11448350
- DOI: 10.3389/fneur.2024.1466275
Recognizing Leber's Hereditary Optic Neuropathy to avoid delayed diagnosis and misdiagnosis
Abstract
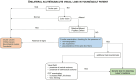
Leber's Hereditary Optic Neuropathy (LHON) is a maternally inherited optic nerve disease primarily caused by mutations in mitochondrial DNA (mtDNA). The peak of onset is typically between 15 and 30 years, but variability exists. Misdiagnosis, often as inflammatory optic neuritis, delays treatment, compounded by challenges in timely genetic diagnosis. Given the availability of a specific treatment for LHON, its early diagnosis is imperative to ensure therapeutic appropriateness. This work gives an updated guidance about LHON differential diagnosis to clinicians dealing also with multiple sclerosi and neuromyelitis optica spectrtum disorders-related optic neuritis. LHON diagnosis relies on clinical signs and paraclinical evaluations. Differential diagnosis in the acute phase primarily involves distinguishing inflammatory optic neuropathies, considering clinical clues such as ocular pain, fundus appearance and visual recovery. Imaging analysis obtained with Optical Coherence Tomography (OCT) assists clinicians in early recognition of LHON and help avoiding misdiagnosis. Genetic testing for the three most common LHON mutations is recommended initially, followed by comprehensive mtDNA sequencing if suspicion persists despite negative results. We present and discuss crucial strategies for accurate diagnosis and management of LHON cases.
Keywords: Leber’s Hereditary Optic Neuropathy; Optical Coherence Tomography; optic nerve; retinal ganglion cell; visual field.
Copyright © 2024 La Morgia, Cascavilla, De Negri, Romano, Canalini, Rossi, Centonze and Filippi.
Conflict of interest statement
CM declares to have been a consultant for Chiesi Farmaceutici, Gensight Biologics, Regulatory PharmaNet and Thenewway srl; CM received speaker honoraria and/or financial support for meetings from Santhera Pharmaceuticals, Chiesi Farmaceutici, Gensight Biologics, Regulatory PharmaNet, Thenewway srl, First Class srl and Biologix. CM is PI/SI for clinical trials sponsored GenSight Biologics, Santhera Pharmaceuticals, Stoke therapeutics, Reneo and OMEICOS therapeutics. FC was employed by Chiesi Italia S.p.A. ADN received speaker honoraria and/or financial support for meetings from Chiesi Farmaceutici and Gensight Biologics. MLC eceived speaker honoraria and/or financial support for meetings from Chiesi Farmaceutici. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources

