COPD associated pulmonary hypertension: A post hoc analysis of the PERFECT study
- PMID: 39364449
- PMCID: PMC11446833
- DOI: 10.1002/pul2.12430
COPD associated pulmonary hypertension: A post hoc analysis of the PERFECT study
Abstract
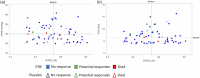
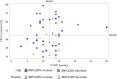
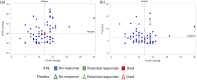
The PERFECT study, a randomized, controlled, double-blind study of inhaled treprostinil in patients with COPD and associated pulmonary hypertension (PH-COPD) was a negative trial that was terminated early. The reason(s) for the negative outcome remains uncertain. A post hoc analysis of data from the PERFECT study was undertaken to identify adverse responders and possibly potential responders. The goal was also to provide insight into phenotypes for possible inclusion and exclusion in future PH-COPD clinical trials. An adverse response on active treatment was seen in 36.4% (24/66) of the subjects compared to 27.6% (16/58) on placebo. There was no evidence to suggest that hyperinflation, bronchospasm, or occult heart failure played any role in the untoward outcomes of the study. The patients who died during the study all had baseline diffusing capacity for carbon monoxide ≤25% of predicted. Evidence of a potential response was seen in 10.6% (7/66) of the patients who received inhaled treprostinil. Patients who had evidence of a treatment response had a baseline mean pulmonary artery pressure of ≥40 mmHg and a forced expiratory volume in the first second of ≥40%. Change in N-terminal prohormone of brain natriuretic peptide did not predict clinical response. This post hoc analysis provides information that may potentially enable improved selection of patients for future therapeutic trials in PH-COPD. These analyses are post hoc, observational, and exploratory. The thresholds defining the spectrum of responders are preliminary and may require further refinement and validation in future studies.
Keywords: chronic obstructive; double‐blind method; hypertension; pulmonary; pulmonary disease; treprostinil.
© 2024 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Steven D. Nathan, Aaron Waxman, Todd Bull and Victor Tapson are paid consultants for Lung Biotechnology and United Therapeutics. Victoria Lacasse, Heidi Bell, Prakash Sista, and Michael Di Marino are employees of United Therapeutics/Lung Biotechnology, the PERFECT study sponsor.
Figures

References
-
- Nathan SD, Argula R, Trivieri MG, Aziz S, Gay E, Medarov B, Parambil J, Raina A, Risbano MG, Thenappan T, Soto JS, Bell H, Lacasse V, Sista P, Di Marino M, Smart A, Hawkes B, Nelson E, Bull T, Tapson V, Waxman A. Inhaled treprostinil in pulmonary hypertension associated with COPD: PERFECT study results. Eur Respir J. 2024;63(6):2400172. 10.1183/13993003.00172-2024 - DOI - PMC - PubMed
-
- Nathan SD, Deng C, King CS, DuBrock HM, Elwing J, Rajagopal S, Rischard F, Sahay S, Broderick M, Shen E, Smith P, Tapson VF, Waxman AB. Inhaled treprostinil dosage in pulmonary hypertension associated with interstitial lung disease and its effects on clinical outcomes. Chest. 2023;163(2):398–406. - PMC - PubMed
LinkOut - more resources
Full Text Sources

