Melatonin Mitigates Cisplatin-Induced Submandibular Gland Damage by Inhibiting Oxidative Stress, Inflammation, Apoptosis, and Fibrosis
- PMID: 39364499
- PMCID: PMC11447767
- DOI: 10.7759/cureus.68515
Melatonin Mitigates Cisplatin-Induced Submandibular Gland Damage by Inhibiting Oxidative Stress, Inflammation, Apoptosis, and Fibrosis
Abstract
Background: The study aims to examine the possible effect of melatonin against cisplatin-induced submandibular degeneration in experimental rats exploring its ameliorative mechanisms.
Methods: Rats were classified into four experimental groups; control group; melatonin group; cisplatin group; and cisplatin+melatonin group. Submandibular tissues were collected. Biochemical, histopathological, and immunohistopathological examination and quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis were performed.
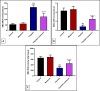
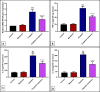
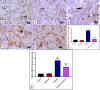
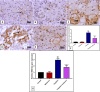
Results: The results indicate that intraperitoneal administration of melatonin (30 mg/kg body weight) alongside cisplatin significantly elevated submandibular glands (SMG) and reduced glutathione (GSH) and superoxide dismutase (SOD) levels (p < 0.001), while it reduced malondialdehyde (MDA) levels, NF-κB gene expression, the protein level of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), immunoexpression of low-dose cyclooxygenase-2 (Cox-2), and CD68. Moreover, melatonin reduced immune and gene expression of alpha-smooth muscle actin (α-SMA), immunoexpression of caspase-3, and gene expression of Bax in comparison to the cisplatin group.
Conclusion: Melatonin attenuated cisplatin-induced submandibular destruction alleviating SMG oxidative stress, inflammation, and fibrosis in addition to halting cellular apoptosis, sheds light on its usage in clinical application.
Keywords: apoptosis; cisplatin; fibrosis; inflammation; melatonin; oxidative stress; submandibular gland.
Copyright © 2024, Badawy et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: The study was conducted in accordance with the Canadian Council on Animal Care Guidelines and approved by the Committee of Research Ethics, Kafrelsheikh University Issued protocol number KFS-IACUC/204/2024. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures




Similar articles
-
Zingerone ameliorates cisplatin-induced ovarian and uterine toxicity via suppression of sex hormone imbalances, oxidative stress, inflammation and apoptosis in female wistar rats.Biomed Pharmacother. 2018 Jun;102:517-530. doi: 10.1016/j.biopha.2018.03.119. Epub 2018 Mar 26. Biomed Pharmacother. 2018. PMID: 29587238
-
Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats.Environ Sci Pollut Res Int. 2019 Aug;26(22):22562-22574. doi: 10.1007/s11356-019-05505-3. Epub 2019 Jun 4. Environ Sci Pollut Res Int. 2019. PMID: 31165450
-
Nephroprotective Effects of Saponins from Leaves of Panax quinquefolius against Cisplatin-Induced Acute Kidney Injury.Int J Mol Sci. 2017 Jul 13;18(7):1407. doi: 10.3390/ijms18071407. Int J Mol Sci. 2017. PMID: 28703736 Free PMC article.
-
Melatonin attenuates oxidative stress, liver damage and hepatocyte apoptosis after bile-duct ligation in rats.Toxicol Ind Health. 2014 Oct;30(9):835-44. doi: 10.1177/0748233712464811. Epub 2012 Oct 24. Toxicol Ind Health. 2014. PMID: 23095487
-
Diallyl sulfide alleviates cisplatin-induced nephrotoxicity in rats via suppressing NF-κB downstream inflammatory proteins and p53/Puma signalling pathway.Clin Exp Pharmacol Physiol. 2018 Jun;45(6):591-601. doi: 10.1111/1440-1681.12910. Epub 2018 Feb 6. Clin Exp Pharmacol Physiol. 2018. PMID: 29266336
Cited by
-
Melatonin: a natural guardian in cancer treatment.Front Pharmacol. 2025 Jul 18;16:1617508. doi: 10.3389/fphar.2025.1617508. eCollection 2025. Front Pharmacol. 2025. PMID: 40756978 Free PMC article. Review.
-
Poly lactic-co-glycolic acid enhances the efficacy of the phytomedicine chrysin against cisplatin induced toxicity in submandibular salivary glands.Sci Rep. 2025 Mar 25;15(1):10262. doi: 10.1038/s41598-025-93112-3. Sci Rep. 2025. PMID: 40133531 Free PMC article.
References
-
- Phase I/II trial of chemotherapy with docetaxel, cisplatin, and S-1 for unresectable advanced squamous cell carcinoma of the esophagus. Ojima T, Nakamura M, Nakamori M, et al. Oncology. 2018;95:116–120. - PubMed
-
- Histological and immunohistochemical study to evaluate the effects of chamomile versus green tea extracts on the salivary glands of methotrexate treated male albino rats (Doctoral dissertation, Fayoum University) Abdulmonem AM. https://www.fayoum.edu.eg/english/Med/Histology/pdf/DrAlshimaaPhdE.pdf Nat Sci. 2020;18:74–95.
LinkOut - more resources
Full Text Sources
Research Materials
