A comparative study of the developability of full-length antibodies, fragments, and bispecific formats reveals higher stability risks for engineered constructs
- PMID: 39364796
- PMCID: PMC11457596
- DOI: 10.1080/19420862.2024.2403156
A comparative study of the developability of full-length antibodies, fragments, and bispecific formats reveals higher stability risks for engineered constructs
Abstract
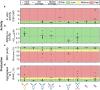
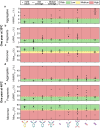
Engineered antibody formats, such as antibody fragments and bispecifics, have the potential to offer improved therapeutic efficacy compared to traditional full-length monoclonal antibodies (mAbs). However, the translation of these non-natural molecules into successful therapeutics can be hampered by developability challenges. Here, we systematically analyzed 64 different antibody constructs targeting Tumor Necrosis Factor (TNF) which cover 8 distinct molecular format families, encompassing full-length antibodies, various types of single chain variable fragments, and bispecifics. We measured 15 biophysical properties related to activity, manufacturing, and stability, scoring variants with a flag-based risk approach and a recent in silico developability profiler. Our comparative assessment revealed that overall developability is higher for the natural full-length antibody format. Bispecific antibodies, antibodies with scFv fragments at the C-terminus of the light chain, and single-chain Fv antibody fragments (scFvs) have intermediate developability properties, while more complicated formats, such as scFv- scFv, bispecific mAbs with one Fab exchanged with a scFv, and diabody formats are collectively more challenging. In particular, our study highlights the propensity for fragmentation and aggregation, both in bulk and at interfaces, for many current engineered formats.
Keywords: Antibody; Biophysics; Colloidal stability; Formulation; Fragmentation; Self-association; Shelf-life stability; aggregation; antibody formats; bispecifics; developability; fragments; scFv.
Conflict of interest statement
N.L., T.E., J.R.B., S.G., Z.C., A.B., are present employees of Novo Nordisk A/S and are shareholders of Novo Nordisk A/S.
Figures




References
-
- Antibody therapeutics approved or in regulatory review in the EU or US . The antibody society. [accessed 2024 May 31]. https://www.antibodysociety.org/resources/approved-antibodies/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
