Antibiotic tolerance among clinical isolates: mechanisms, detection, prevalence, and significance
- PMID: 39364999
- PMCID: PMC11629620
- DOI: 10.1128/cmr.00106-24
Antibiotic tolerance among clinical isolates: mechanisms, detection, prevalence, and significance
Abstract
SUMMARYAntibiotic treatment failures in the absence of resistance are not uncommon. Recently, attention has grown around the phenomenon of antibiotic tolerance, an underappreciated contributor to recalcitrant infections first detected in the 1970s. Tolerance describes the ability of a bacterial population to survive transient exposure to an otherwise lethal concentration of antibiotic without exhibiting resistance. With advances in genomics, we are gaining a better understanding of the molecular mechanisms behind tolerance, and several studies have sought to examine the clinical prevalence of tolerance. Attempts have also been made to assess the clinical significance of tolerance through in vivo infection models and prospective/retrospective clinical studies. Here, we review the data available on the molecular mechanisms, detection, prevalence, and clinical significance of genotypic tolerance that span ~50 years. We discuss the need for standardized methodology and interpretation criteria for tolerance detection and the impact that methodological inconsistencies have on our ability to accurately assess the scale of the problem. In terms of the clinical significance of tolerance, studies suggest that tolerance contributes to worse outcomes for patients (e.g., higher mortality, prolonged hospitalization), but historical data from animal models are varied. Furthermore, we lack the necessary information to effectively treat tolerant infections. Overall, while the tolerance field is gaining much-needed traction, the underlying clinical significance of tolerance that underpins all tolerance research is still far from clear and requires attention.
Keywords: animal models; antibiotic tolerance; clinical microbiology; diagnostics; treatment failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

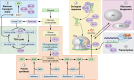


References
-
- Ikuta KS, Swetschinski LR, Robles Aguilar G, Sharara F, Mestrovic T, Gray AP, Davis Weaver N, Wool EE, Han C, Gershberg Hayoon A. 2022. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet 400:2221–2248. doi: 10.1016/S0140-6736(22)02185-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

