High-quality genome of a novel Thermosynechococcaceae species from Namibia and characterization of its protein expression patterns at elevated temperatures
- PMID: 39365014
- PMCID: PMC11450739
- DOI: 10.1002/mbo3.70000
High-quality genome of a novel Thermosynechococcaceae species from Namibia and characterization of its protein expression patterns at elevated temperatures
Abstract
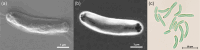
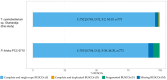
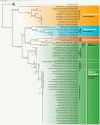
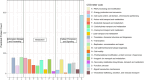
Thermophilic cyanobacteria thrive in extreme environments, making their thermoresistant enzymes valuable for industrial applications. Common habitats include hot springs, which act as evolutionary accelerators for speciation due to geographical isolation. The family Thermosynechococcaceae comprises thermophilic cyanobacteria known for their ability to thrive in high-temperature environments. These bacteria are notable for their photosynthetic capabilities, significantly contributing to primary production in extreme habitats. Members of Thermosynechococcaceae exhibit unique adaptations that allow them to perform photosynthesis efficiently at elevated temperatures, making them subjects of interest for studies on microbial ecology, evolution, and potential biotechnological applications. In this study, the genome of a thermophilic cyanobacterium, isolated from a hot spring near Okahandja in Namibia, was sequenced using a PacBio Sequel IIe long-read platform. Cultivations were performed at elevated temperatures of 40, 50, and 55°C, followed by proteome analyses based on the annotated genome. Phylogenetic investigations, informed by the 16S rRNA gene and aligned nucleotide identity (ANI), suggest that the novel cyanobacterium is a member of the family Thermosynechococcaceae. Furthermore, the new species was assigned to a separate branch, potentially representing a novel genus. Whole-genome alignments supported this finding, revealing few conserved regions and multiple genetic rearrangement events. Additionally, 129 proteins were identified as differentially expressed in a temperature-dependent manner. The results of this study broaden our understanding of cyanobacterial adaptation to extreme environments, providing a novel high-quality genome of Thermosynechococcaceae cyanobacterium sp. Okahandja and several promising candidate proteins for expression and characterization studies.
Keywords: Thermosynechococcaceae; cyanobacteria; genomics; proteomics; taxonomy; thermophilic.
© 2024 The Author(s). MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures






References
-
- Abed, R. M. M. , Dobretsov, S. , & Sudesh, K. (2009). Applications of Cyanobacteria in biotechnology. Journal of Applied Microbiology, 106(1), 1–12. - PubMed
-
- Afgan, E. , Baker, D. , van den Beek, M. , Blankenberg, D. , Bouvier, D. , Čech, M. , Chilton, J. , Clements, D. , Coraor, N. , Eberhard, C. , Grüning, B. , Guerler, A. , Hillman‐Jackson, J. , Von Kuster, G. , Rasche, E. , Soranzo, N. , Turaga, N. , Taylor, J. , Nekrutenko, A. , & Goecks, J. (2016). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2016 update. Nucleic Acids Research, 44(W1), W3–W10. - PMC - PubMed
-
- Aimi, J. , Qiu, H. , Williams, J. , Zalkin, H. , & Dixon, J. E. (1990). De novo purine nucleotide biosynthesis: Cloning of human and avian cDNAs encoding the trifunctional glycinamide ribonucleotide synthetase‐aminoimidazole ribonucleotide synthetase‐glycinamide ribonucleotide transformylase by functional complementation in E. coli . Nucleic Acids Research, 18(22), 6665–6672. - PMC - PubMed
-
- Aksu, Z. , Ertuğrul, S. , & Dönmez, G. (2009). Single and binary chromium(VI) and Remazol Black B biosorption properties of Phormidium sp. Journal of Hazardous Materials, 168(1), 310–318. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

