Pseudomonas aeruginosa kills Staphylococcus aureus in a polyphosphate-dependent manner
- PMID: 39365057
- PMCID: PMC11520310
- DOI: 10.1128/msphere.00686-24
Pseudomonas aeruginosa kills Staphylococcus aureus in a polyphosphate-dependent manner
Abstract
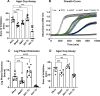
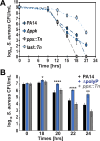
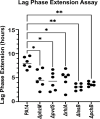
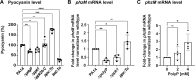
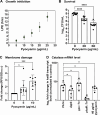
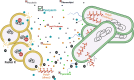
Due to their frequent coexistence in many polymicrobial infections, including in patients with cystic fibrosis or burn/chronic wounds, many studies have investigated the mechanistic details of the interaction between the opportunistic pathogens Pseudomonas aeruginosa and Staphylococcus aureus. P. aeruginosa rapidly outcompetes S. aureus under in vitro cocultivation conditions, which is mediated by several of P. aeruginosa's virulence factors. Here, we report that polyphosphate (polyP), an efficient stress defense system and virulence factor in P. aeruginosa, plays a role in the pathogen's ability to inhibit and kill S. aureus in a contact-independent manner. We show that P. aeruginosa cells characterized by low polyP levels are less detrimental to S. aureus growth and survival while the Gram-positive pathogen is significantly more compromised by the presence of P. aeruginosa cells that produce high levels of polyP. The polyP-dependent phenotype of P. aeruginosa-mediated killing of S. aureus could at least in part be direct, as polyP was detected in the spent media and causes significant damage to the S. aureus cell envelope. However, more likely is that polyP's effects are indirect through modulating the production of one of P. aeruginosa's virulence factors, pyocyanin. We show that pyocyanin production in P. aeruginosa occurs polyP-dependently and harms S. aureus through membrane damage and potentially the generation of reactive oxygen species, resulting in the increased expression of antioxidant enzymes. In summary, our study adds a new component to the list of biomolecules that the Gram-negative pathogen P. aeruginosa generates to compete with S. aureus for resources.IMPORTANCEHow do interactions between microorganisms shape the course of polymicrobial infections? Previous studies have provided evidence that the two opportunistic pathogens Pseudomonas aeruginosa and Staphylococcus aureus generate molecules that modulate their interaction with potentially significant impact on disease outcomes. Our study identified the biopolymer polyphosphate (polyP) as a new effector molecule that impacts P. aeruginosa's interaction with S. aureus. We show that P. aeruginosa kills S. aureus in a polyP-dependent manner, which occurs primarily through the polyP-dependent production of the P. aeruginosa virulence factor pyocyanin. Our findings add a new role for polyP to an already extensive list of functions. A more in-depth understanding of how polyP influences interspecies interactions is critical, as targeting polyP synthesis in bacteria such as P. aeruginosa may have a significant impact on other microorganisms and potentially result in dynamic changes in the microbial composition.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; oxidative stress; phenazine; polymicrobial interactions; polyphosphate; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Update of
-
Pseudomonas aeruginosa kills Staphylococcus aureus in a polyphosphate-dependent manner.bioRxiv [Preprint]. 2023 Dec 6:2023.12.05.570291. doi: 10.1101/2023.12.05.570291. bioRxiv. 2023. Update in: mSphere. 2024 Oct 29;9(10):e0068624. doi: 10.1128/msphere.00686-24. PMID: 38106195 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
