A Remote Patient Monitoring System With Feedback Mechanisms Using a Smartwatch: Concept, Implementation, and Evaluation Based on the activeDCM Randomized Controlled Trial
- PMID: 39365164
- PMCID: PMC11624455
- DOI: 10.2196/58441
A Remote Patient Monitoring System With Feedback Mechanisms Using a Smartwatch: Concept, Implementation, and Evaluation Based on the activeDCM Randomized Controlled Trial
Abstract
Background: Technological advances allow for recording and sharing health-related data in a patient-centric way using smartphones and wearables. Secure sharing of such patient-generated data with physicians would enable close management of individual health trajectories, monitoring of risk factors, and asynchronous feedback. However, most remote patient monitoring (RPM) systems currently available are not fully integrated into hospital IT systems or lack a patient-centric design.
Objective: The objective of this study was to conceptualize and implement a user-friendly, reusable, interoperable, and secure RPM system incorporating asynchronous feedback mechanisms using a broadly available consumer wearable (Apple Watch). In addition, this study sought to evaluate factors influencing patient acceptance of such systems.
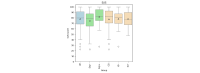
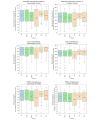
Methods: The RPM system requirements were established through focus group sessions. Subsequently, a system concept was designed and implemented using an iterative approach ensuring technical feasibility from the beginning. To assess clinical feasibility, the system was used as part of the activeDCM prospective randomized interventional study focusing on dilated cardiomyopathy. Each patient used the system for at least 12 months. The System Usability Scale was used to measure usability from a subjective patient perspective. In addition, an evaluation was conducted on the objective wearable interaction frequency as well as the completeness of transmitted data classified into sensor-based health data (SHD) and patient-reported outcome measures (PROMs). Descriptive statistics using box plots and bootstrapped multiple linear regression with 95% CIs were used for evaluation analyzing the influence of age, sex, device experience, and intervention group membership.
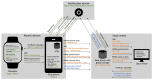
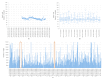
Results: The RPM system comprised 4 interoperable components: patient devices, a data server, a data viewer, and a notification service. The system was evaluated with 95 consecutive patients with dilated cardiomyopathy (28/95, 29% female; mean age 50, SD 12 y) who completed the activeDCM study protocol. The system's app achieved a mean System Usability Scale score of 78 (SD 17), which was most influenced by device experience. In total, 87% (83/95) of the patients could integrate the use of the app well or very well into their daily routine, and 71% (67/95) saw a benefit of the RPM system for management of their health condition. On average, patients interacted with the wearable on 61% (SD 26%) of days enrolled in the study. SHD were available on average for 78% (SD 23%) of days, and PROM data were available on 64% (SD 27%) of weeks enrolled in the study. Wearable interaction frequency, SHD, and PROM completeness were most influenced by intervention group membership.
Conclusions: Our results mark a first step toward integrating RPM systems based on a consumer wearable device for primary patient input into standardized clinical workflows. They can serve as a blueprint for creating a user-friendly, reusable, interoperable, and secure RPM system that can be integrated into patients' daily routines.
Keywords: HL7 FHIR; Health Level 7 Fast Healthcare Interoperability Resources; cardiology; consumer device; dilated cardiomyopathy; heart failure; mobile health; mobile phone; remote patient monitoring; telemedicine; usability; wearable.
©Reto Wettstein, Farbod Sedaghat-Hamedani, Oliver Heinze, Ali Amr, Christoph Reich, Theresa Betz, Elham Kayvanpour, Angela Merzweiler, Christopher Büsch, Isabell Mohr, Birgit Friedmann-Bette, Norbert Frey, Martin Dugas, Benjamin Meder. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 22.11.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



References
-
- Izmailova ES, Wagner JA, Perakslis ED. Wearable devices in clinical trials: hype and hypothesis. Clin Pharmacol Ther. 2018 Jul 02;104(1):42–52. doi: 10.1002/cpt.966. https://europepmc.org/abstract/MED/29205294 - DOI - PMC - PubMed
-
- Ringeval M, Wagner G, Denford J, Paré G, Kitsiou S. Fitbit-based interventions for healthy lifestyle outcomes: systematic review and meta-analysis. J Med Internet Res. 2020 Oct 12;22(10):e23954. doi: 10.2196/23954. https://www.jmir.org/2020/10/e23954/ v22i10e23954 - DOI - PMC - PubMed
-
- Hershman SG, Bot BM, Shcherbina A, Doerr M, Moayedi Y, Pavlovic A, Waggott D, Cho MK, Rosenberger ME, Haskell WL, Myers J, Champagne MA, Mignot E, Salvi D, Landray M, Tarassenko L, Harrington RA, Yeung AC, McConnell MV, Ashley EA. Physical activity, sleep and cardiovascular health data for 50,000 individuals from the MyHeart counts study. Sci Data. 2019 Apr 11;6(1):24. doi: 10.1038/s41597-019-0016-7. https://doi.org/10.1038/s41597-019-0016-7 10.1038/s41597-019-0016-7 - DOI - DOI - PMC - PubMed
-
- Rising CJ, Gaysynsky A, Blake KD, Jensen RE, Oh A. Willingness to share data from wearable health and activity trackers: analysis of the 2019 health information national trends survey data. JMIR Mhealth Uhealth. 2021 Dec 13;9(12):e29190. doi: 10.2196/29190. https://mhealth.jmir.org/2021/12/e29190/ v9i12e29190 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

