S100 as marker for immune effector cell-associated neurotoxicity syndrome
- PMID: 39365474
- PMCID: PMC12370851
- DOI: 10.1007/s00508-024-02451-0
S100 as marker for immune effector cell-associated neurotoxicity syndrome
Abstract
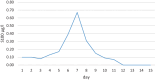
Chimeric antigen receptor (CAR)-T cell therapy is a new and successful treatment for otherwise refractory malignancies but despite the growing number of applications, this form of treatment is still associated with significant toxicity. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) in particular are common and dangerous side effects. This report is about two patients who received CAR‑T cell therapy and subsequently developed ICANS. This was successfully treated. During CAR‑T cell therapy, a blood marker, S100, was monitored daily. It correlated with the occurrence and progression of ICANS.
Keywords: Chimeric antigen receptor (CAR)-T cell therapy; Marker; Neurology; Side effects; Toxicity.
© 2024. The Author(s).
Conflict of interest statement
Conflict of interest: A. Schulenburg, L.Z. Rüsing, A. Bumberger, M. Mitterbauer and W. Rabitsch declare that they have no competing interests.
Figures
References
-
- June CH. Next generation CAR T cells for lymphoma and beyond. Hematol Oncol. 2019;37:S2–S34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources