Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion
- PMID: 39365817
- PMCID: PMC11474043
- DOI: 10.1073/pnas.2401071121
Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion
Abstract
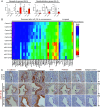
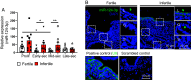
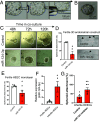
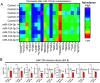
The endometrium undergoes substantial remodeling in each menstrual cycle to become receptive to an implanting embryo. Abnormal endometrial receptivity is one of the major causes of embryo implantation failure and infertility. MicroRNA-124-3p is elevated in both the serum and endometrial tissue of women with chronic endometritis, a condition associated with infertility. MicroRNA-124-3p also has a role in cell adhesion, a key function during receptivity to allow blastocysts to adhere and implant. In this study, we aimed to determine the function of microRNA-124-3p on endometrial epithelial adhesive capacity during receptivity and effect on embryo implantation. Using a unique inducible, uterine epithelial-specific microRNA overexpression mouse model, we demonstrated that elevated uterine epithelial microRNA-124-3p impaired endometrial receptivity by altering genes associated with cell adhesion and polarity. This resulted in embryo implantation failure. Similarly in a second mouse model, increasing microRNA-124-3p expression only in mouse uterine surface (luminal) epithelium impaired receptivity and led to implantation failure. In humans, we demonstrated that microRNA-124-3p was abnormally increased in the endometrial epithelium of women with unexplained infertility during the receptive window. MicroRNA-124-3p overexpression in primary human endometrial epithelial cells (HEECs) impaired primary human embryo trophectoderm attachment in a 3-dimensional culture model of endometrium. Reduction of microRNA-124-3p in HEECs from infertile women normalized HEEC adhesive capacity. Overexpression of microRNA-124-3p or knockdown of its direct target IQGAP1 reduced fertile HEEC adhesion and its ability to lose polarity. Collectively, our data highlight that microRNA-124-3p and its protein targets contribute to endometrial receptivity by altering cell polarity and adhesion.
Keywords: embryo implantation; endometrial epithelium; endometrial receptivity; miR-124-3p; microRNA.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Wilcox A. J., Baird D. D., Weinberg C. R., Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 340, 1796–1799 (1999). - PubMed
-
- Kao L., et al. , Global gene profiling in human endometrium during the window of implantation. Endocrinology 143, 2119–2138 (2002). - PubMed
-
- Strowitzki T., Germeyer A., Popovici R., Von Wolff M., The human endometrium as a fertility-determining factor. Hum. Reprod. Update 12, 617–630 (2006). - PubMed
-
- Psychoyos A., Uterine receptivity for nidation. Ann. N. Y. Acad. Sci. 476, 36–42 (1986). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

