Inflammation and Immunity in Liver Neoplasms: Implications for Future Therapeutic Strategies
- PMID: 39365846
- PMCID: PMC11794036
- DOI: 10.1158/1535-7163.MCT-23-0726
Inflammation and Immunity in Liver Neoplasms: Implications for Future Therapeutic Strategies
Abstract
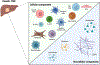
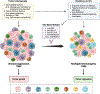
Over the past two decades, the "hallmarks of cancer" have revolutionized cancer research and highlighted the crucial roles of inflammation and immunity. Protumorigenic inflammation promotes cancer development along with inhibition of antitumor immunity, shaping the tumor microenvironment (TME) toward a tumor-permissive state and further enhancing the malignant potential of cancer cells. This immunosuppressive TME allows tumors to evade immunosurveillance. Thus, understanding the complex interplay between tumors and the immune system within the TME has become pivotal, especially with the advent of immunotherapy. Although immunotherapy has achieved notable success in many malignancies, primary liver cancer, particularly hepatocellular carcinoma, presents unique challenges. The hepatic immunosuppressive environment poses obstacles to the effectiveness of immunotherapy, along with high mortality rates and limited treatment options for patients with liver cancer. In this review, we discuss current understanding of the complex immune-mediated mechanisms underlying liver neoplasms, focusing on hepatocellular carcinoma and liver metastases. We describe the molecular and cellular heterogeneity within the TME, highlighting how this presents unique challenges and opportunities for immunotherapy in liver cancers. By unraveling the immune landscape of liver neoplasms, this review aims to contribute to the development of more effective therapeutic interventions, ultimately improving clinical outcomes for patients with liver cancer.
©2024 American Association for Cancer Research.
Conflict of interest statement
The authors declare they have nothing to disclose.
Figures

References
-
- Hanahan D Hallmarks of Cancer: New Dimensions. Cancer Discov 2022;12(1):31–46 doi 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

