BCL-XL-targeting antibody-drug conjugates are active in preclinical models and mitigate on-mechanism toxicity of small-molecule inhibitors
- PMID: 39365864
- PMCID: PMC11451551
- DOI: 10.1126/sciadv.ado7120
BCL-XL-targeting antibody-drug conjugates are active in preclinical models and mitigate on-mechanism toxicity of small-molecule inhibitors
Abstract
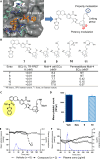
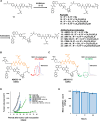
Overexpression of the antiapoptotic protein B-cell lymphoma-extra large (BCL-XL) is associated with drug resistance and disease progression in numerous cancers. The compelling nature of this protein as a therapeutic target prompted efforts to develop selective small-molecule BCL-XL inhibitors. Although efficacious in preclinical models, we report herein that selective BCL-XL inhibitors cause severe mechanism-based cardiovascular toxicity in higher preclinical species. To overcome this liability, antibody-drug conjugates were constructed using altered BCL-XL-targeting warheads, unique linker technologies, and therapeutic antibodies. The epidermal growth factor receptor-targeting antibody-drug conjugate AM1-15 inhibited growth of tumor xenografts and did not cause cardiovascular toxicity nor dose-limiting thrombocytopenia in monkeys. While an unprecedented BCL-XL-mediated toxicity was uncovered in monkey kidneys upon repeat dosing of AM1-15, this toxicity was mitigated via further drug-linker modification to afford AM1-AAA (AM1-25). The AAA drug-linker has since been incorporated into mirzotamab clezutoclax, the first selective BCL-XL-targeting agent to enter human clinical trials.
Figures




References
-
- Hanahan D., Weinberg R. A., Hallmarks of cancer: The next generation. Cell 144, 646–674 (2011). - PubMed
-
- Khan N., Kahl B., Targeting BCL-2 in hematologic malignancies. Target. Oncol. 13, 257–267 (2018). - PubMed
-
- Amundson S. A., Myers T. G., Scudiero D., Kitada S., Reed J. C., Fornace A. J. Jr., An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60, 6101–6110 (2000). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

