Shine and darkle the blood vessels: Multiparameter hypersensitive MR angiography for diagnosis of panvascular diseases
- PMID: 39365870
- PMCID: PMC11451532
- DOI: 10.1126/sciadv.adq4082
Shine and darkle the blood vessels: Multiparameter hypersensitive MR angiography for diagnosis of panvascular diseases
Abstract
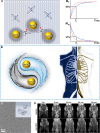
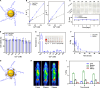
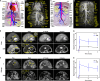
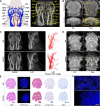
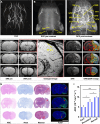
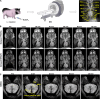
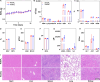
Magnetic resonance angiography (MRA) is pivotal for diagnosing panvascular diseases. However, single-modality MRA falls short in diagnosing diverse vascular abnormalities. Thus, contrast agents combining T1 and T2 effects are sought for multiparameter MRA with clinical promise, yet achieving a balance in T1 and T2 contrast enhancement effects remains a scientific challenge. Herein, we developed a hypersensitive multiparameter MRA strategy using dual-modality NaGdF4 nanoparticles. Because of the longer tumbling time (τR), NaGdF4 nanoparticles can improve the longitudinal relaxivity (r1), brightening vessels in T1-weighted sequences. Simultaneously, the regular arrangement of Gd3+ in the crystal induces magnetic anisotropy, creating local static magnetic field heterogeneity and generating negative signals in T2-weighted sequences. Consequently, the efficacy of NaGdF4-enhanced high-resolution multiparameter MRA has been validated in diagnosing ischemic stroke and Alzheimer's disease in rodent models. In addition, the dual-contrast imaging has been realized on swine with a clinical 3.0-T magnetic resonance imaging scanner, highly emphasizing the clinical translation prospect.
Figures

References
-
- Suowen X., Iqra I., Peter J. L., Hong L., Danielle K., Xueying Z., Sihui L., Zhuoming L., Peiqing L., Jihong H., Ian C. H., Eno E. E., Scott J. C., Alastair G. S., Jianping W., Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: From mechanism to pharmacotherapies. Pharmacol. Rev. 73, 924–967 (2021). - PubMed
-
- Hu Y., Zhao Y., Li P., Lu H., Li H., Ge J., Hypoxia and panvascular diseases: Exploring the role of hypoxia-inducible factors in vascular smooth muscle cells under panvascular pathologies. Sci. Bull. 68, 1954–1974 (2023). - PubMed
-
- Musialek P., Montauk L., Saugnet A., Micari A., Hopkins L. N., The cardio-vascular future of panvascular medicine: The basics. Kardiol. Pol. 77, 899–901 (2019). - PubMed
-
- Guthaner D. F., Wexler L., Enzmann D. R., Riederer S. J., Keyes G. S., Collins W. F., Brody W. R., Evaluation of peripheral vascular disease using digital subtraction angiography. Radiology 147, 393–398 (1983). - PubMed
-
- Singh A., Mor-Avi V., Patel A. R., The role of computed tomography myocardial perfusion imaging in clinical practice. J. Cardiovasc. Comput. 14, 185–194 (2020). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

