Insight into the Rate-Determining Step in Photocatalytic Z-Scheme Overall Water Splitting by Employing A Series of Perovskite RTaON2 (R = Pr, Nd, Sm, and Gd) as Model Photocatalysts
- PMID: 39365918
- PMCID: PMC11669088
- DOI: 10.1021/jacs.4c08001
Insight into the Rate-Determining Step in Photocatalytic Z-Scheme Overall Water Splitting by Employing A Series of Perovskite RTaON2 (R = Pr, Nd, Sm, and Gd) as Model Photocatalysts
Abstract
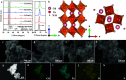
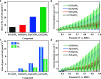
Photocatalysis is an intricate process that involves a multitude of physical and chemical factors operating across diverse temporal and spatial scales. Identifying the dominant factors that influence photocatalyst performance is one of the central challenges in the field. Here, we synthesized a series of perovskite RTaON2 semiconductors with different A-site rare earth atoms (R = Pr, Nd, Sm, and Gd) as model photocatalysts to discuss the influence of the A-site modulation on their local structures as well as both physical and chemical properties and to get insight into the rate-determining step in photocatalytic Z-scheme overall water splitting (OWS). It is interesting to find that, with a decreasing ionic radius of the A-site cations, the RTaON2 compounds exhibit continuous blue shift of light absorption and a concomitant reduction in the lifetime of photogenerated carriers, revealing a significant influence of A-site atoms on the light absorption and charge separation processes. On the other hand, the A-site atomic substitution was revealed to significantly modulate the valence band positions as well as surface oxidation kinetics. By employing the Pt-modified RTaON2 as H2-evolving photocatalysts, the activity of photocatalytic Z-scheme OWS for hydrogen production on them is found to be determined by its surface oxidation process instead of light absorption or charge separation. Our results give the first experimental demonstration of the rate-determining step during the photocatalytic Z-scheme OWS processes, as should be instructive for the design and development of other efficient solar-to-chemical energy conversion systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Nishioka S.; Osterloh F. E.; Wang X.; Mallouk T. E.; Maeda K. Photocatalytic water splitting. Nat. Rev. Methods Primers 2023, 3 (1), 42. 10.1038/s43586-023-00226-x. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

