Mechanism of action and impact of thiol homeostasis on efficacy of an enzyme replacement therapy for classical homocystinuria
- PMID: 39366068
- PMCID: PMC11489331
- DOI: 10.1016/j.redox.2024.103383
Mechanism of action and impact of thiol homeostasis on efficacy of an enzyme replacement therapy for classical homocystinuria
Abstract
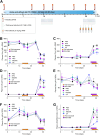
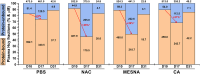
Homocystinuria (HCU) due to cystathionine beta-synthase (CBS) deficiency is characterized by elevated plasma and tissue homocysteine levels. There is no cure, but HCU is typically managed by methionine/protein restriction and vitamin B6 supplementation. Enzyme replacement therapy (ERT) based on human CBS has been developed and has shown significant efficacy correcting HCU phenotype in several mouse models by bringing plasma total homocysteine below the clinically relevant 100 μM threshold. As the reactive nature of homocysteine promotes disulfide formation and protein binding, and ERT is unable to normalize plasma total homocysteine levels, the mechanism of action of ERT in HCU remains to be further characterized. Here we showed that only a reduced homocysteine serves as a substrate for CBS and its availability restricts the homocysteine-degrading capacity of CBS. We also demonstrated that cells export homocysteine in its reduced form, which is efficiently metabolized by CBS in the culture medium. Availability of serine, a CBS co-substrate, was not a limiting factor in our cell-based model. Biological reductants, such as N-acetylcysteine, MESNA or cysteamine, increased the availability of the reduced homocysteine and thus promoted its subsequent CBS-based elimination. In a transgenic I278T mouse model of HCU, administration of biological reductants significantly increased the proportion of protein-unbound homocysteine in plasma, which improved the efficacy of the co-administered CBS-based ERT, as evidenced by significantly lower plasma total homocysteine levels. These results clarify the mechanism of action of CBS-based ERT and unveil novel pharmacological approaches to further increase its efficacy.
Keywords: Cystathionine beta-synthase; Disulfide isomerization; Enzyme replacement therapy; Homocysteine; Homocystinuria; N-acetylcysteine; Plasma redox status.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest TM is an inventor on patents related to pegtibatinase, provides ad-hoc consulting to Travere Therapeutics and receives research support from Travere Therapeutics. TB receives compensation for metabolomic analyses from Travere Therapeutics. WC, KL and SR are current or former employees and stockholders of Travere Therapeutics, which clinically develops pegtibatinase as an enzyme replacement therapy for classical homocystinuria. TMP and CS declare no conflicting interests.
Figures




References
-
- Mudd S.H., Levy H.L., Kraus J.P. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Kinzler K., Vogelstein B., editors. McGraw-Hill; New York: 2001. Disorders of transsulfuration; pp. 2007–2056.
-
- Brosnan J.T., Brosnan M.E., Bertolo R.F.P., Brunton J.A. Methionine: a metabolically unique amino acid. Livest. Sci. 2007;112:2–7.
-
- Jakubowski H. Pathophysiological consequences of homocysteine excess. J. Nutr. 2006;136:1741S–1749S. - PubMed
-
- Barber G.W., Spaeth G.L. The successful treatment of homocystinuria with pyridoxine. J. Pediatr. 1969;75:463–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

