Construction of self-driving anti-αFR CAR-engineered NK cells based on IFN-γ and TNF-α synergistically induced high expression of CXCL10
- PMID: 39366148
- PMCID: PMC11489333
- DOI: 10.1016/j.neo.2024.101065
Construction of self-driving anti-αFR CAR-engineered NK cells based on IFN-γ and TNF-α synergistically induced high expression of CXCL10
Abstract
Introduction: Ovarian cancer is the most malignant gynecological tumor. Previous studies have demonstrated that chimeric antigen receptor (CAR)-engineered NK-92 cells targeting folate receptor α (αFR) (NK-92-αFR-CAR) can specifically kill αFR-positive ovarian cancer cells. However, the migration barrier restricts antitumor effects of CAR-engineered cells.
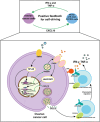
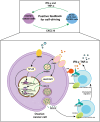
Objectives: To elucidate the mechanism by which NK-92-αFR-CAR cells induce the secretion of chemokine CXCL10 during killing ovarian cancer cells. It is speculated that NK-92-αFR-CAR-CXCR3A can target αFR and have chemotaxis of CXCL10, and they may have stronger killing effect of ovarian cancer.
Methods: Study the mechanism of CXCL10 expression strongly induced by TNF-α and IFN-γ combined stimulation in ovarian cancer cells. Construct the fourth generation of NK-92-αFR-CAR-CXCR3A cells, which were co-expressed CXCR3A and αFR-CAR. Evaluate the killing and migration effects of NK-92-αFR-CAR-CXCR3A in vitro and in vivo.
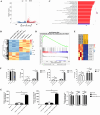
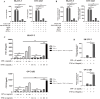
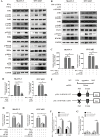
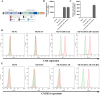
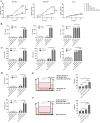
Results: RNA sequencing (RNA-seq) first revealed that the expression level of the chemokine CXCL10 was most significantly increased in ovarian cancer cells co-cultured with NK-92-αFR-CAR. Secondly, cytokine stimulation experiments confirmed that IFN-γ and TNF-α secreted by NK-92-αFR-CAR synergistically induced high CXCL10 expression in ovarian cancer cells. Further signaling pathway experiments showed that IFN-γ and TNF-α enhanced the activation level of the IFN-γ-IFNGR-JAK1/2-STAT1-CXCL10 signaling axis. Cytotoxicity experiments showed that NK-92-αFR-CAR-CXCR3A cells could not only efficiently kill αFR-positive ovarian cancer cells in vitro but also secrete IFN-γ and TNF-α. Higher migration than that of NK-92-αFR-CAR was detected in NK-92-αFR-CAR-CXCR3A using transwell assay. NK-92-αFR-CAR-CXCR3A effectively killed tumor cells in different mouse xenograft models of ovarian cancer and increased infiltration into tumor tissue.
Conclusion: This study confirmed that IFN-γ and TNF-α secreted by αFR-CAR-engineered NK cells can synergistically induce high expression of CXCL10 in ovarian cancer cells and constructed self-driving αFR-CAR-engineered NK cells that can break through migration barriers based on CXCL10, which may provide a new therapeutic weapon for ovarian cancer.
Keywords: CAR-NK; CXCL10; CXCR3; Immunotherapy; Ovarian cancer.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors have declared no conflict of interest
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

