Self-assembling nanoparticle engineered from the ferritinophagy complex as a rabies virus vaccine candidate
- PMID: 39366932
- PMCID: PMC11452399
- DOI: 10.1038/s41467-024-52908-z
Self-assembling nanoparticle engineered from the ferritinophagy complex as a rabies virus vaccine candidate
Abstract
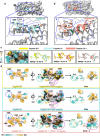
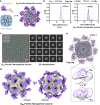
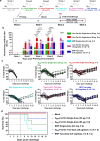
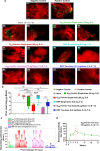
Over the past decade, there has been a growing interest in ferritin-based vaccines due to their enhanced antigen immunogenicity and favorable safety profiles, with several vaccine candidates targeting various pathogens advancing to phase I clinical trials. Nevertheless, challenges associated with particle heterogeneity, improper assembly and unanticipated immunogenicity due to the bulky protein adaptor have impeded further advancement. To overcome these challenges, we devise a universal ferritin-adaptor delivery platform based on structural insights derived from the natural ferritinophagy complex of the human ferritin heavy chain (FTH1) and the nuclear receptor coactivator 4 (NCOA4). The engineered ferritinophagy (Fagy)-tag peptide demonstrate significantly enhanced binding affinity to the 24-mer ferritin nanoparticle, enabling efficient antigen presentation. Subsequently, we construct a self-assembling rabies virus (RABV) vaccine candidate by noncovalently conjugating the Fagy-tagged glycoprotein domain III (GDIII) of RABV to the ferritin nanoparticle, maintaining superior homogeneity, stability and immunogenicity. This vaccine candidate induces potent, rapid, and durable immune responses, and protects female mice against the authentic RABV challenge after single-dose administration. Furthermore, this universal, ferritin-based antigen conjugating strategy offers significant potential for developing vaccine against diverse pathogens and diseases.
© 2024. The Author(s).
Conflict of interest statement
Y.G., D.F., and P.Y. declare the following competing interests: patent has been filed for the Fagy-tag and self-assembly nanoparticle system presented here. All other authors declare no competing interests.
Figures



Similar articles
-
A nucleoside-modified mRNA vaccine forming rabies virus-like particle elicits strong cellular and humoral immune responses against rabies virus infection in mice.Emerg Microbes Infect. 2024 Dec;13(1):2389115. doi: 10.1080/22221751.2024.2389115. Epub 2024 Aug 14. Emerg Microbes Infect. 2024. PMID: 39129566 Free PMC article.
-
A single dose of an ALVAC vector-based RABV virus-like particle candidate vaccine induces a potent immune response in mice, cats and dogs.Emerg Microbes Infect. 2024 Dec;13(1):2406280. doi: 10.1080/22221751.2024.2406280. Epub 2024 Sep 30. Emerg Microbes Infect. 2024. PMID: 39295522 Free PMC article.
-
Toward the Development of a Pan-Lyssavirus Vaccine.Viruses. 2024 Jul 10;16(7):1107. doi: 10.3390/v16071107. Viruses. 2024. PMID: 39066269 Free PMC article.
-
Rabies vaccine development by expression of recombinant viral glycoprotein.Arch Virol. 2017 Feb;162(2):323-332. doi: 10.1007/s00705-016-3128-9. Epub 2016 Oct 31. Arch Virol. 2017. PMID: 27796547 Review.
-
Research Advances on the Interactions between Rabies Virus Structural Proteins and Host Target Cells: Accrued Knowledge from the Application of Reverse Genetics Systems.Viruses. 2021 Nov 16;13(11):2288. doi: 10.3390/v13112288. Viruses. 2021. PMID: 34835093 Free PMC article. Review.
Cited by
-
Screening of host proteins interacting with the African swine fever virus outer membrane protein CD2v.Front Microbiol. 2025 Jun 30;16:1585335. doi: 10.3389/fmicb.2025.1585335. eCollection 2025. Front Microbiol. 2025. PMID: 40661982 Free PMC article.
-
Progress and Challenges in HIV-1 Vaccine Research: A Comprehensive Overview.Vaccines (Basel). 2025 Jan 31;13(2):148. doi: 10.3390/vaccines13020148. Vaccines (Basel). 2025. PMID: 40006695 Free PMC article. Review.
-
Optimization of Conditions for Expression of Dengue Serotype 2 EDIII Protein in Escherichia coli and Immune Responses of Adjuvant-Free EDIII Ferritin Nanoparticles Against Dengue Virus in BALB/c Mice.Viruses. 2025 Jan 17;17(1):129. doi: 10.3390/v17010129. Viruses. 2025. PMID: 39861918 Free PMC article.
-
Advances in protein subunit vaccines against H1N1/09 influenza.Front Immunol. 2024 Nov 22;15:1499754. doi: 10.3389/fimmu.2024.1499754. eCollection 2024. Front Immunol. 2024. PMID: 39650643 Free PMC article. Review.
References
-
- Munro, H. N. & Linder, M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol. Rev.58, 317–396 (1978). - PubMed
-
- Harrison, P. M. & Arosio, P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta (BBA) Bioenerg.1275, 161–203 (1996). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

