Postnatal supplementation with alarmins S100a8/a9 ameliorates malnutrition-induced neonate enteropathy in mice
- PMID: 39366940
- PMCID: PMC11452687
- DOI: 10.1038/s41467-024-52829-x
Postnatal supplementation with alarmins S100a8/a9 ameliorates malnutrition-induced neonate enteropathy in mice
Abstract
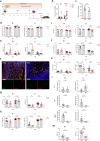
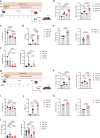
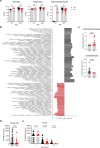
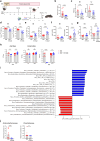
Malnutrition is linked to 45% of global childhood mortality, however, the impact of maternal malnutrition on the child's health remains elusive. Previous studies suggested that maternal malnutrition does not affect breast milk composition. Yet, malnourished children often develop a so-called environmental enteropathy, assumed to be triggered by frequent pathogen uptake and unfavorable gut colonization. Here, we show in a murine model that maternal malnutrition induces a persistent inflammatory gut dysfunction in the offspring that establishes during nursing and does not recover after weaning onto standard diet. Early intestinal influx of neutrophils, impaired postnatal development of gut-regulatory functions, and expansion of Enterobacteriaceae were hallmarks of this enteropathy. This gut phenotype resembled those developing under deficient S100a8/a9-supply via breast milk, which is a known key factor for the postnatal development of gut homeostasis. We could confirm that S100a8/a9 is lacking in the breast milk of malnourished mothers and the offspring's intestine. Nutritional supply of S100a8 to neonates of malnourished mothers abrogated the aberrant development of gut mucosal immunity and microbiota colonization and protected them lifelong against severe enteric infections and non-infectious bowel diseases. S100a8 supplementation after birth might be a promising measure to counteract deleterious imprinting of gut immunity by maternal malnutrition.
© 2024. The Author(s).
Conflict of interest statement
F.G. is the founder of MV BioTherapeutics, a company developing mucosal vaccines and biotherapeutics. All remaining authors declare no conflicts of interest.
Figures


Similar articles
-
S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants.Gastroenterology. 2020 Dec;159(6):2130-2145.e5. doi: 10.1053/j.gastro.2020.08.019. Epub 2020 Aug 15. Gastroenterology. 2020. PMID: 32805279
-
Role of S100A8/A9 for Cytokine Secretion, Revealed in Neutrophils Derived from ER-Hoxb8 Progenitors.Int J Mol Sci. 2021 Aug 17;22(16):8845. doi: 10.3390/ijms22168845. Int J Mol Sci. 2021. PMID: 34445548 Free PMC article.
-
Inhibition of S100A8/A9 ameliorates neuroinflammation by blocking NET formation following traumatic brain injury.Redox Biol. 2025 Apr;81:103532. doi: 10.1016/j.redox.2025.103532. Epub 2025 Feb 5. Redox Biol. 2025. PMID: 39929053 Free PMC article.
-
S100A8/A9 in Inflammation.Front Immunol. 2018 Jun 11;9:1298. doi: 10.3389/fimmu.2018.01298. eCollection 2018. Front Immunol. 2018. PMID: 29942307 Free PMC article. Review.
-
Pathogenic roles of neutrophil-derived alarmins (S100A8/A9) in heart failure: From molecular mechanisms to therapeutic insights.Br J Pharmacol. 2023 Mar;180(5):573-588. doi: 10.1111/bph.15998. Epub 2022 Dec 26. Br J Pharmacol. 2023. PMID: 36464854 Review.
References
-
- WHO. Fact sheets - Malnutrition. https://www.who.int/news-room/fact-sheets/detail/malnutrition (2021).
-
- Black, R. E. et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet371, 243–260 (2008). - PubMed
-
- UNICEF/WHO/WB The UNICEF/WHO/WB Joint Child Malnutrition Estimates (JME) group released new data for 2021. https://www.who.int/news/item/06-05-2021-the-unicef-who-wb-joint-child-m... (2021).
-
- UNICEF. As the war in Ukraine continues, millions of children In the Middle East and North Africa at increased risk of malnutrition amid food price hikes.https://www.unicef.org/press-releases/war-ukraine-continues-millions-chi... (2022).
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- INV-004078/GATES/Gates Foundation/United States
- INV-004078/GATES/Gates Foundation/United States
- 01EK2103A/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- 01EK2103D/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- VI 538/6-3, VI 538-9-1, 492620490, 491676693, 390874280/Deutsche Forschungsgemeinschaft (German Research Foundation)

