Alternate-day fasting delays pubertal development in normal-weight mice but prevents high-fat diet-induced obesity and precocious puberty
- PMID: 39366955
- PMCID: PMC11452675
- DOI: 10.1038/s41387-024-00335-w
Alternate-day fasting delays pubertal development in normal-weight mice but prevents high-fat diet-induced obesity and precocious puberty
Abstract
Background/objectives: Childhood obesity, particularly in girls, is linked to early puberty onset, heightening risks for adult-onset diseases. Addressing childhood obesity and precocious puberty is vital to mitigate societal burdens. Despite existing costly and invasive medical interventions, introducing lifestyle-based alternatives is essential. Our study investigates alternate-day fasting's (ADF) impact on pubertal development in normal-weight and high-fat diet (HFD)-induced obese female mice.
Methods: Four groups of female mice were utilized, with dams initially fed control chow during and before pregnancy. Post-parturition, two groups continued on control chow, while two switched to an HFD. Offspring diets mirrored maternal exposure. One control and one HFD group were subjected to ADF. Morphometry and hormone analyses at various time points were performed.
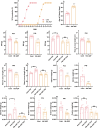
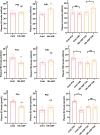
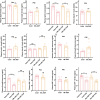
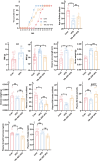
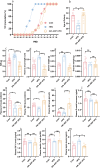
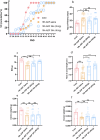
Results: Our findings demonstrate that ADF in normal-weight mice led to reduced body length, weight, uterine, and ovarian weights, accompanied by delayed puberty and lower levels of sex hormones and growth hormone (GH). Remarkably, GH treatment effectively prevented ADF-induced growth reduction but did not prevent delayed puberty. Conversely, an HFD increased body length, induced obesity and precocious puberty, and altered sex hormones and leptin levels, which were counteracted by ADF regimen. Our data indicate ADF's potential in managing childhood obesity and precocious puberty.
Conclusions: ADF reduced GH and sex hormone levels, contributing to reduced growth and delayed puberty, respectively. Therefore, parents of normal-weight children should be cautious about prolonged overnight fasting. ADF prevented HFD-induced obesity and precocious puberty, offering an alternative to medical approaches; nevertheless, further studies are needed for translation into clinical practice.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Postnatal feeding with high-fat diet induces obesity and precocious puberty in C57BL/6J mouse pups: a novel model of obesity and puberty.Front Med. 2017 Jun;11(2):266-276. doi: 10.1007/s11684-017-0530-y. Epub 2017 May 13. Front Med. 2017. PMID: 28500430
-
Comparative Impact of Alternate-Day Fasting and Time-Restricted Feeding on Placental Function and Fetal Development in Maternal Obesity.Nutrients. 2024 Dec 25;17(1):25. doi: 10.3390/nu17010025. Nutrients. 2024. PMID: 39796458 Free PMC article.
-
Alternate-day fasting protects the livers of mice against high-fat diet-induced inflammation associated with the suppression of Toll-like receptor 4/nuclear factor κB signaling.Nutr Res. 2016 Jun;36(6):586-93. doi: 10.1016/j.nutres.2016.02.001. Epub 2016 Feb 16. Nutr Res. 2016. PMID: 27188904
-
Diagnosis and management of precocious sexual maturation: an updated review.Eur J Pediatr. 2021 Oct;180(10):3073-3087. doi: 10.1007/s00431-021-04022-1. Epub 2021 Mar 21. Eur J Pediatr. 2021. PMID: 33745030 Review.
-
Disorders of Puberty: An Approach to Diagnosis and Management.Am Fam Physician. 2017 Nov 1;96(9):590-599. Am Fam Physician. 2017. PMID: 29094880 Review.
Cited by
-
Gut flora influences the hypothalamic-gonadal axis to regulate the pathogenesis of obesity-associated precocious puberty.Sci Rep. 2024 Nov 21;14(1):28844. doi: 10.1038/s41598-024-80140-8. Sci Rep. 2024. PMID: 39572735 Free PMC article.
-
Obesity: pathophysiology and therapeutic interventions.Mol Biomed. 2025 Apr 25;6(1):25. doi: 10.1186/s43556-025-00264-9. Mol Biomed. 2025. PMID: 40278960 Free PMC article. Review.
References
-
- Ullah R, Shen Y, Zhou YD, Fu J. Perinatal metabolic inflammation in the hypothalamus impairs the development of homeostatic feeding circuitry. Metabolism. 2023;147:155677. - PubMed
-
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94:4953–60. - PubMed
-
- Liang X, Huang K, Dong G, Chen R, Chen S, Zheng R, et al. Current pubertal development in Chinese children and the impact of overnutrition, lifestyle and perinatal factors. J Clin Endocrinol Metab. 2023;108:2282–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

