Evolution of the conformational dynamics of the molecular chaperone Hsp90
- PMID: 39366960
- PMCID: PMC11452706
- DOI: 10.1038/s41467-024-52995-y
Evolution of the conformational dynamics of the molecular chaperone Hsp90
Abstract
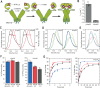
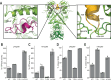
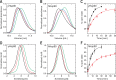
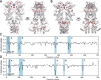
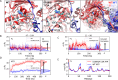
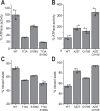
Hsp90 is a molecular chaperone of central importance for protein homeostasis in the cytosol of eukaryotic cells, with key functional and structural traits conserved from yeast to man. During evolution, Hsp90 has gained additional functional importance, leading to an increased number of interacting co-chaperones and client proteins. Here, we show that the overall conformational transitions coupled to the ATPase cycle of Hsp90 are conserved from yeast to humans, but cycle timing as well as the dynamics are significantly altered. In contrast to yeast Hsp90, the human Hsp90 is characterized by broad ensembles of conformational states, irrespective of the absence or presence of ATP. The differences in the ATPase rate and conformational transitions between yeast and human Hsp90 are based on two residues in otherwise conserved structural elements that are involved in triggering structural changes in response to ATP binding. The exchange of these two mutations allows swapping of the ATPase rate and of the conformational transitions between human and yeast Hsp90. Our combined results show that Hsp90 evolved to a protein with increased conformational dynamics that populates ensembles of different states with strong preferences for the N-terminally open, client-accepting states.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Importance of cycle timing for the function of the molecular chaperone Hsp90.Nat Struct Mol Biol. 2016 Nov;23(11):1020-1028. doi: 10.1038/nsmb.3305. Epub 2016 Oct 10. Nat Struct Mol Biol. 2016. PMID: 27723736 Free PMC article.
-
Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle.J Biol Chem. 2004 Dec 10;279(50):51989-98. doi: 10.1074/jbc.M410562200. Epub 2004 Oct 2. J Biol Chem. 2004. PMID: 15466438
-
The conserved NxNNWHW motif in Aha-type co-chaperones modulates the kinetics of Hsp90 ATPase stimulation.Nat Commun. 2019 Mar 20;10(1):1273. doi: 10.1038/s41467-019-09299-3. Nat Commun. 2019. PMID: 30894538 Free PMC article.
-
The chaperone Hsp90: changing partners for demanding clients.Trends Biochem Sci. 2013 May;38(5):253-62. doi: 10.1016/j.tibs.2013.02.003. Epub 2013 Mar 16. Trends Biochem Sci. 2013. PMID: 23507089 Review.
-
The 'active life' of Hsp90 complexes.Biochim Biophys Acta. 2012 Mar;1823(3):614-23. doi: 10.1016/j.bbamcr.2011.07.020. Epub 2011 Aug 4. Biochim Biophys Acta. 2012. PMID: 21840346 Free PMC article. Review.
Cited by
-
More than Just Protein Folding: The Epichaperome, Mastermind of the Cancer Cell.Cells. 2025 Jan 30;14(3):204. doi: 10.3390/cells14030204. Cells. 2025. PMID: 39936995 Free PMC article. Review.
-
The known unknowns of the Hsp90 chaperone.Elife. 2024 Dec 31;13:e102666. doi: 10.7554/eLife.102666. Elife. 2024. PMID: 39737863 Free PMC article. Review.
-
Biochemistry of Heat Shock Proteins From Human Intracellular Protozoan Parasites as Diagnostic and Therapeutic Biomarkers.Biochemistry. 2025 Jun 17;64(12):2529-2543. doi: 10.1021/acs.biochem.5c00120. Epub 2025 Jun 2. Biochemistry. 2025. PMID: 40452612 Free PMC article. Review.
-
Chaperone-Mediated Responses and Mitochondrial-Endoplasmic Reticulum Coupling: Emerging Insight into Alzheimer's Disease.Cells. 2025 Jul 31;14(15):1179. doi: 10.3390/cells14151179. Cells. 2025. PMID: 40801612 Free PMC article. Review.
-
Metabolic engineering of yeast to efficiently synthesize heme and hemoproteins: recent advance and prospects.FEMS Yeast Res. 2025 Jan 30;25:foaf019. doi: 10.1093/femsyr/foaf019. FEMS Yeast Res. 2025. PMID: 40228812 Free PMC article. Review.
References
-
- Richter, K., Haslbeck, M. & Buchner, J. The heat shock response: life on the verge of death. Mol. Cell40, 253–266 (2010). - PubMed
-
- Karagoz, G. E. & Rudiger, S. G. Hsp90 interaction with clients. Trends Biochem. Sci.40, 117–125 (2015). - PubMed
-
- Zhao, R. et al. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell120, 715–727 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

