Plateau depolarizations in spontaneously active neurons detected by calcium or voltage imaging
- PMID: 39367010
- PMCID: PMC11452489
- DOI: 10.1038/s41598-024-70319-4
Plateau depolarizations in spontaneously active neurons detected by calcium or voltage imaging
Abstract
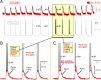
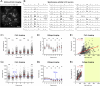
In calcium imaging studies, Ca2+ transients are commonly interpreted as neuronal action potentials (APs). However, our findings demonstrate that robust optical Ca2+ transients primarily stem from complex "AP-Plateaus", while simple APs lacking underlying depolarization envelopes produce much weaker photonic signatures. Under challenging in vivo conditions, these "AP-Plateaus" are likely to surpass noise levels, thus dominating the Ca2+ recordings. In spontaneously active neuronal culture, optical Ca2+ transients (OGB1-AM, GCaMP6f) exhibited approximately tenfold greater amplitude and twofold longer half-width compared to optical voltage transients (ArcLightD). The amplitude of the ArcLightD signal exhibited a strong correlation with the duration of the underlying membrane depolarization, and a weaker correlation with the presence of a fast sodium AP. Specifically, ArcLightD exhibited robust responsiveness to the slow "foot" but not the fast "trunk" of the neuronal AP. Particularly potent stimulators of optical signals in both Ca2+ and voltage imaging modalities were APs combined with plateau potentials (AP-Plateaus), resembling dendritic Ca2+ spikes or "UP states" in pyramidal neurons. Interestingly, even the spikeless plateaus (amplitude > 10 mV, duration > 200 ms) could generate conspicuous Ca2+ optical signals in neurons. Therefore, in certain circumstances, Ca2+ transients should not be interpreted solely as indicators of neuronal AP firing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Cohen, E., Ivenshitz, M., Amor-Baroukh, V., Greenberger, V. & Segal, M. Determinants of spontaneous activity in networks of cultured hippocampus. Brain Res.1235, 21–30 (2008). - PubMed
-
- Larkum, M. E., Nevian, T., Sandler, M., Polsky, A. & Schiller, J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: A new unifying principle. Science325(5941), 756–760 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

