Design, synthesis, and biological evaluation of novel imidazole derivatives as analgesic and anti-inflammatory agents: experimental and molecular docking insights
- PMID: 39367036
- PMCID: PMC11452658
- DOI: 10.1038/s41598-024-72399-8
Design, synthesis, and biological evaluation of novel imidazole derivatives as analgesic and anti-inflammatory agents: experimental and molecular docking insights
Erratum in
-
Author Correction: Design, synthesis, and biological evaluation of novel imidazole derivatives as analgesic and anti-inflammatory agents: experimental and molecular docking insights.Sci Rep. 2024 Oct 25;14(1):25392. doi: 10.1038/s41598-024-76905-w. Sci Rep. 2024. PMID: 39455865 Free PMC article. No abstract available.
Abstract
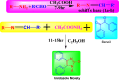
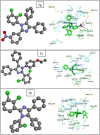
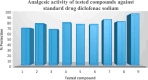
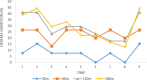
Imidazole moieties exhibit a broad range of biological activities, including analgesic, anti-depressant, anticancer, anti-fungal, anti-tubercular, anti-inflammatory, antimicrobial, antiviral, and antifungal properties. In this study, we explored the use of Schiff base for the synthesis of new imidazole derivatives as anti-inflammatory and pain-relieving agents. A series of eight novel imidazole analogues (2a-h) were prepared in three steps with excellent yields. All compounds were characterized using IR, NMR, and mass spectral data. Their analgesic and anti-inflammatory activities were evaluated using hot plate and paw oedema methods. Compound 2 g (1-(2,3-dichlorophenyl)-2-(3-nitrophenyl)-4,5-diphenyl-1H-imidazole) showed significant analgesic activity (89% at 100 mg/kg b.w.), while compounds 2a (2-(2,6-dichlorophenyl)-1-(4-ethoxyphenyl)-4,5-diphenyl-1H-imidazole) and 2b (2-(2,3-dichlorophenyl)-1-(2-chlorophenyl)-4,5-diphenyl-1H-imidazole) exhibited good anti-inflammatory activity (100% at 100 mg/kg b.w.), comparable to diclofenac salt (100% at 50 mg/kg b.w.). Molecular docking studies were conducted using Schrödinger software version 2021-2, employing the OPLS4 force field for both receptor and ligand preparation. The results were visualized using molecular visualization software such as PyMOL. These studies revealed that compound 2g exhibited the highest binding affinity with the COX-2 receptor (-5.516 kcal/mol). Compound 2g formed three conventional hydrogen bonds with residues GLN-242 (bond length: 2.3 Å) and ARG-343 (bond lengths: 2.2 Å & 2.4 Å). This binding affinity was comparable to that of Diclofenac salt, which showed the highest binding affinity of -5.627 kcal/mol with the COX-2 receptor. Diclofenac salt formed two conventional hydrogen bonds with the residues ARG-344 (bond length: 2.0 Å) and TRP-140 (bond length: 1.7 Å). Later, molecular dynamic simulations confirmed the stable binding affinity of compound 2g with the protein. Furthermore, other compounds also demonstrated potential binding to the receptor-binding pocket region. The anti-inflammatory potential of the synthesized compounds was evaluated using the carrageenan-induced rat hind paw oedema model, while the analgesic potential was assessed using the hot plate method. These evaluations were conducted in comparison with Diclofenac sodium, serving as the standard compound. However, compound 2g stood out for its superior analgesic activity, as confirmed by in-vivo examination. These findings suggest that these novel imidazole derivatives have potential as anti-inflammatory and analgesic agents.
Keywords: Analgesic activity; Anti-inflammatory agents; COX-2 inhibition; Diclofenac sodium; Imidazole derivatives; Molecular docking studies; Schiff base synthesis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

