Midostaurin added to 10-day decitabine, for patients unfit for intensive chemotherapy with AML and higher risk MDS, irrespective of FLT3 mutational status, does not improve outcome
- PMID: 39367118
- PMCID: PMC11868317
- DOI: 10.1007/s00277-024-06033-y
Midostaurin added to 10-day decitabine, for patients unfit for intensive chemotherapy with AML and higher risk MDS, irrespective of FLT3 mutational status, does not improve outcome
Abstract
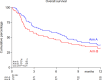
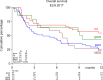
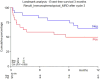
The treatment of older patients with acute myeloid leukemia (AML) considered unfit for receiving intensive chemotherapy is challenging. Based on the hypothesis that addition of the broad tyrosine kinase inhibitor (TKI) midostaurin could improve the response to hypomethylating agents, irrespective of FLT3 gene mutational status, we conducted a randomized phase II multicenter study to assess the tolerability and efficacy of the addition of midostaurin to a 10-day schedule of decitabine in unfit (i.e. Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI) ≥ 3) AML and higher risk myelodysplasia (MDS) patients (HOVON155 trial). In total, 140 eligible patients were randomly (1:1) assigned to treatment with 10-days of decitabine alone (N = 70) or combined with midostaurin (50 mg bid;starting the day following the last dose of decitabine), (N = 70). Addition of midostaurin was well tolerated and the number of AEs was comparable for both treatment arms. Early death rates (< 30 days) were similar as well (10%). In the decitabine plus midostaurin arm 24% reached CR/CRi, the median OS was 4.8 months and 1-yrs OS was 31% which compared with 34% CR/CRi, median OS of 7.4 months and 1-yrs OS of 37% for the decitabine alone group (NS). Thus, while the addition of midostaurin appears safe, it does not enhance therapeutic efficacy of decitabine in unfit AML patients.
Keywords: AML; Decitabine; Midostaurin; Older; Phase II trial.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and informed consent: This study was approved by the Medical Ethical committee of the University Medical Centre Groningen, and all participants provided informed consent in accordance with the Declaration of Helsinki. This investigator-sponsored trial was financially supported by Janssen Pharmaceutics who provided decitabine free of charge and Novartis who supported the trial and provided midostaurin free of charge. Competing interests: The authors declare no competing interests.
Figures
References
-
- Kantarjian HM, Thomas XG, Dmoszynska A et al (2012) Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 30:2670–2677 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

