Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway
- PMID: 39367342
- PMCID: PMC11451247
- DOI: 10.1186/s12872-024-04192-4
Quercetin regulates pulmonary vascular remodeling in pulmonary hypertension by downregulating TGF-β1-Smad2/3 pathway
Abstract
Background: Pulmonary arterial hypertension (PAH) is a worldwide challenging disease characterized by progressive elevation of pulmonary artery pressure. The proliferation, migration and phenotypic transformation of pulmonary smooth muscle cells are the key steps of pulmonary vascular remodeling. Quercetin (3,3', 4', 5, 6-pentahydroxyflavone, Que) is a natural flavonol compound that has antioxidant, anti-inflammatory, anti-tumor and other biological activities. Studies have shown that Que has therapeutic effects on PAH. However, the effect of quercetin on pulmonary vascular remodeling in PAH and its mechanism remain unclear.
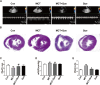
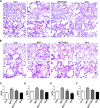
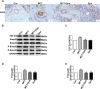
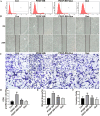
Methods and results: In vivo, PAH rats were constructed by intraperitoneal injection of monocrotaline (MCT) at 60 mg/kg. Human pulmonary artery smooth muscle cells (HPASMCs) were treated with platelet-derived growth factor BB (PDGF-BB) 20 ng/mL to construct PAH cell model in vitro. The results showed that in vivo studies, MCT could induce right ventricular wall hyperplasia, narrow the small and medium pulmonary artery cavity, up-regulate the expression of proliferating and migration-related proteins proliferating cell nuclear antigen (PCNA) and osteopontin (OPN), and down-regulate the expression of alpha-smooth muscle actin (α-SMA). Que reversed the MCT-induced results. This process works by down-regulating the transforming growth factor-β1 (TGF-β1)/ Smad2/3 signaling pathway. In vitro studies, Que had the same effect on PDGF-BB-induced proliferation and migration cell models.
Conclusions: Que inhibits the proliferation, migration and phenotypic transformation of HPASMCs by down-regulating TGF-β1/Smad2/Smad3 pathway, thereby reducing right ventricular hyperplasia (RVH) and pulmonary vascular remodeling, providing potential pharmacological and molecular explanations for the treatment of PAH.
Keywords: Migration; PAH; Proliferation; Que; TGF-β1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Lei S, Peng F, Li ML, et al. LncRNA-SMILR modulates RhoA/ROCKsignaling by targeting miR-141 to regulate vascular remodeling in pulmonaryarterial hypertension. Am J Physiol Heart Circ Physiol. 2020;319:H377–91. - PubMed
-
- Courboulin A, Barrier M, Perreault T, et al. Plumbaginreverses proliferation and resistance to apoptosis in experimental PAH. EurRespir J. 2012;40:618–29. - PubMed
-
- Wang SC. PCNA: a silent housekeeper or a potential therapeutic target. Trends Pharmacol Sci. 2014;35:178–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

