Interleukin-2 improves insulin sensitivity through hypothalamic sympathetic activation in obese mice
- PMID: 39367382
- PMCID: PMC11453069
- DOI: 10.1186/s12974-024-03244-y
Interleukin-2 improves insulin sensitivity through hypothalamic sympathetic activation in obese mice
Abstract
Background: IL-2 regulates T cell differentiation: low-dose IL-2 induces immunoregulatory Treg differentiation, while high-dose IL-2 acts as a potent activator of cytotoxic T cells and NK cells. Therefore, high-dose IL-2 has been studied for use in cancer immunotherapy. We aimed to utilize low-dose IL-2 to treat inflammatory diseases such as obesity and insulin resistance, which involve low-grade chronic inflammation.
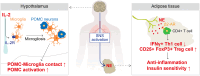
Main body: Systemic administration of low-dose IL-2 increased Treg cells and decreased inflammation in gonadal white adipose tissue (gWAT), leading to improved insulin sensitivity in high-fat diet-fed obese mice. Additionally, central administration of IL-2 significantly enhanced insulin sensitivity through the activation of the sympathetic nervous system. The sympathetic signaling induced by central IL-2 administration not only decreased interferon γ (IFNγ) + Th1 cells and the expression of pro-inflammatory cytokines, including Il-1β, Il-6, and Il-8, but also increased CD4 + CD25 + FoxP3 + Treg cells and Tgfβ expression in the gWAT of obese mice. These phenomena were accompanied by hypothalamic microgliosis and activation of pro-opiomelanocortin neurons. Furthermore, sympathetic denervation in gWAT reversed the enhanced insulin sensitivity and immune cell polarization induced by central IL-2 administration.
Conclusion: Overall, we demonstrated that IL-2 improves insulin sensitivity through two mechanisms: direct action on CD4 + T cells and via the neuro-immune axis triggered by hypothalamic microgliosis.
Keywords: Adipose tissue inflammation; Hypothalamic microglia; Insulin resistance; Interleukin-2; Pro-opiomelanocortin (POMC) neurons; Sympathetic nervous system.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–92. 10.1056/NEJM198512053132327. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

