Visceral adiposity in postmenopausal women is associated with a pro-inflammatory gut microbiome and immunogenic metabolic endotoxemia
- PMID: 39367431
- PMCID: PMC11453046
- DOI: 10.1186/s40168-024-01901-1
Visceral adiposity in postmenopausal women is associated with a pro-inflammatory gut microbiome and immunogenic metabolic endotoxemia
Abstract
Background: Obesity, and in particular abdominal obesity, is associated with an increased risk of developing a variety of chronic diseases. Obesity, aging, and menopause are each associated with differential shifts in the gut microbiome. Obesity causes chronic low-grade inflammation due to increased lipopolysaccharide (LPS) levels which is termed "metabolic endotoxemia." We examined the association of visceral adiposity tissue (VAT) area, circulating endotoxemia markers, and the gut bacterial microbiome in a cohort of aged postmenopausal women.
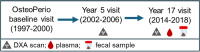
Methods: Fifty postmenopausal women (mean age 78.8 ± 5.3 years) who had existing adipose measurements via dual x-ray absorptiometry (DXA) were selected from the extremes of VAT: n = 25 with low VAT area (45.6 ± 12.5 cm2) and n = 25 with high VAT area (177.5 ± 31.3 cm2). Dietary intake used to estimate the Healthy Eating Index (HEI) score was assessed with a food frequency questionnaire. Plasma LPS, LPS-binding protein (LBP), anti-LPS antibodies, anti-flagellin antibodies, and anti-lipoteichoic acid (LTA) antibodies were measured by ELISA. Metagenomic sequencing was performed on fecal DNA. Female C57BL/6 mice consuming a high-fat or low-fat diet were treated with 0.4 mg/kg diet-derived fecal isolated LPS modeling metabolic endotoxemia, and metabolic outcomes were measured after 6 weeks.
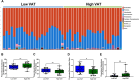
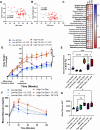
Results: Women in the high VAT group showed increased Proteobacteria abundance and a lower Firmicutes/Bacteroidetes ratio. Plasma LBP concentration was positively associated with VAT area. Plasma anti-LPS, anti-LTA, and anti-flagellin IgA antibodies were significantly correlated with adiposity measurements. Women with high VAT showed significantly elevated LPS-expressing bacteria compared to low VAT women. Gut bacterial species that showed significant associations with both adiposity and inflammation (anti-LPS IgA and LBP) were Proteobacteria (Escherichia coli, Shigella spp., and Klebsiella spp.) and Veillonella atypica. Healthy eating index (HEI) scores negatively correlated with % body fat and anti-LPS IgA antibodies levels. Preclinical murine model showed that high-fat diet-fed mice administered a low-fat diet fecal-derived LPS displayed reduced body weight, decreased % body fat, and improved glucose tolerance test parameters when compared with saline-injected or high-fat diet fecal-derived LPS-treated groups consuming a high-fat diet.
Conclusions: Increased VAT in postmenopausal women is associated with elevated gut Proteobacteria abundance and immunogenic metabolic endotoxemia markers. Low-fat diet-derived fecal-isolated LPS improved metabolic parameters in high-fat diet-fed mice giving mechanistic insights into potential pro-health signaling mediated by under-acylated LPS isoforms. Video Abstract.
Keywords: Aging; Inflammation; Leaky gut; Lipopolysaccharide; Menopause; Metabolic endotoxemia; Microbiome; Obesity; Women’s Health Initiative.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;360:1–8. - PubMed
-
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–3. - PubMed
-
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. - PubMed
-
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. - PubMed
MeSH terms
Substances
Grants and funding
- W81XWH-20-1-0014/Congressionally Directed Medical Research Programs
- W81XWH-20-1-0014/Congressionally Directed Medical Research Programs
- W81XWH-20-1-0014/Congressionally Directed Medical Research Programs
- R01 DE013505, R01 DE024523/DE/NIDCR NIH HHS/United States
- R01 DE013505, R01 DE024523/DE/NIDCR NIH HHS/United States
- R01 DE013505, R01 DE024523/DE/NIDCR NIH HHS/United States
- R01 DE013505, R01 DE024523/DE/NIDCR NIH HHS/United States
- R01 DE013505, R01 DE024523/DE/NIDCR NIH HHS/United States
- HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C/HL/NHLBI NIH HHS/United States
- HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

