GZMA suppressed GPX4-mediated ferroptosis to improve intestinal mucosal barrier function in inflammatory bowel disease
- PMID: 39367435
- PMCID: PMC11451002
- DOI: 10.1186/s12964-024-01836-y
GZMA suppressed GPX4-mediated ferroptosis to improve intestinal mucosal barrier function in inflammatory bowel disease
Abstract
Background: Our previous study has demonstrated a decreased colonic CD8+CD39+ T cells, enrichment of granzyme A (GZMA), was found in pediatric-onset colitis and inflammatory bowel disease (IBD) characterized by impaired intestinal barrier function. However, the influence of GZMA on intestinal barrier function remains unknown.
Methods: Western blotting(WB), real-time PCR (qPCR), immunofluorescence (IF) and in vitro permeability assay combined with intestinal organoid culture were used to detect the effect of GZMA on intestinal epithelial barrier function in vivo and in vitro. Luciferase, immunoprecipitation (IP) and subcellular fractionation isolation were performed to identify the mechanism through which GZMA modulated intestinal epithelial barrier function.
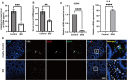
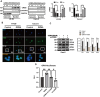
Results: Herein, we, for the first time, demonstrated that CD8+CD39+ T cells promoted intestinal epithelial barrier function through GZMA, leading to induce Occludin(OCLN) and Zonula Occludens-1(ZO-1) expression, which was attributed to enhanced CDX2-mediated cell differentiation caused by increased glutathione peroxidase 4(GPX4)-induced ferroptosis inhibition in vivo and in vitro. Mechanically, GZMA inhibited intestinal epithelial cellular PDE4B activation to trigger cAMP/PKA/CREB cascade signaling to increase CREB nuclear translocation, initiating GPX4 transactivity. In addition, endogenous PKA interacted with CREB, and this interaction was enhanced in response to GZMA. Most importantly, administration of GZMA could alleviate DSS-induced colitis in vivo.
Conclusion: These findings extended the novel insight of GZMA contributed to intestinal epithelial cell differentiation to improve barrier function, and enhacement of GZMA could be a promising strategy to patients with IBD.
Keywords: CDX2; Ferroptosis; GZMA; IBD; Intestinal barrier function.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. - PubMed
-
- Fanning AS, et al. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273(45):29745–53. - PubMed
MeSH terms
Substances
Grants and funding
- No.2020A1515110109/Basic and Applied Basic Research Foundation of Guangdong Province
- No.2021A1515012194/Basic and Applied Basic Research Foundation of Guangdong Province
- 011009003/Guangzhou Clinical Medicine Institute of Pediatric Digestive
- No.82200607/National Natural Science Foundation of China
- No.82070537/National Natural Science Foundation of China
- 202201020631/Basic and applied research project of Guangzhou Municipal Science and Technology Project
- 2023YFC2706500/Accurate diagnosis, treatment and prevention strategies for digestive system diseases related to diarrhea in children
- No.2023A03J0866/Guangzhou key laboratory of Pediatric Inflammatory Bowel Disease
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

