Insights to Ang/Tie signaling pathway: another rosy dawn for treating retinal and choroidal vascular diseases
- PMID: 39367441
- PMCID: PMC11451039
- DOI: 10.1186/s12967-024-05441-y
Insights to Ang/Tie signaling pathway: another rosy dawn for treating retinal and choroidal vascular diseases
Abstract
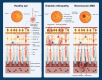
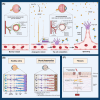
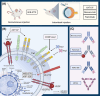
Retinal neurovascular unit (NVU) is a multi-cellular structure that consists of the functional coupling between neural tissue and vascular system. Disrupted NVU will result in the occurrence of retinal and choroidal vascular diseases, which are characterized by the development of neovascularization, increased vascular permeability, and inflammation. This pathological entity mainly includes neovascular age-related macular degeneration (neovascular-AMD), diabetic retinopathy (DR) retinal vein occlusion (RVO), and retinopathy of prematurity (ROP). Emerging evidences suggest that the angopoietin/tyrosine kinase with immunoglobulin and epidermal growth factor homology domains (Ang/Tie) signaling pathway is essential for the development of retinal and choroidal vascular. Tie receptors and their downstream pathways play a key role in modulating the vascular development, vascular stability, remodeling and angiogenesis. Angiopoietin 1 (Ang1) is a natural agonist of Tie2 receptor, which can promote vascular stability. On the other hand, angiopoietin 2 (Ang2) is an antagonist of Tie2 receptor that causes vascular instability. Currently, agents targeting the Ang/Tie signaling pathway have been used to inhibit neovascularization and vascular leakage in neovascular-AMD and DR animal models. Particularly, the AKB-9778 and Faricimab have shown promising efficacy in improving visual acuity in patients with neovascular-AMD and DR. These experimental and clinical evidences suggest that activation of Ang/Tie signaling pathway can inhibit the vascular permeability, neovascularization, thereby maintaining the normal function and structure of NVU. This review seeks to introduce the versatile functions and elucidate the modulatory mechanisms of Ang/Tie signaling pathway. Recent pharmacologic therapies targeting this pathway are also elaborated and summarized. Further translation of these findings may afford a new therapeutic strategy from bench to bedside.
Keywords: Angiopoietin; Clinical translation; Diabetic retinopathy; Neovascular age-related macular degeneration; Vascular endothelial growth factor.
© 2024. The Author(s).
Conflict of interest statement
There are no competing interests.
Figures

References
-
- Thomas CJ, Mirza RG, Gill MK. Age-related Macular Degeneration. Med Clin North Am. 2021;105(3):473–91. - PubMed
-
- Ortiz-Seller A, Martorell P, Barranco H, Pascual-Camps I, Morcillo E, Ortiz JL. Comparison of different agents and doses of anti-vascular endothelial growth factors (aflibercept, bevacizumab, conbercept, ranibizumab) versus laser for retinopathy of prematurity: a network meta-analysis. Surv Ophthalmol. 2024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

