Head-to-head comparison of influenza vaccines in children: a systematic review and meta-analysis
- PMID: 39367499
- PMCID: PMC11453075
- DOI: 10.1186/s12967-024-05676-9
Head-to-head comparison of influenza vaccines in children: a systematic review and meta-analysis
Abstract
Although vaccination is considered the most effective weapon against influenza, coverage rates, national vaccination policies, and funding vary largely around the globe. Despite their huge potential for achieving herd immunity, child-focused national vaccination strategies that favor pain-free nasal vaccines are uncommon. CENTRAL, Embase, and MEDLINE were last searched on November 13, 2023. Active-controlled randomized controlled trials comparing the live-attenuated intranasal vaccine with the inactivated intramuscular influenza vaccine in children were included. Event rates of laboratory-confirmed influenza virus infection, all-cause mortality, hospitalization, serious adverse events, adverse events, and financial outcomes were extracted based on the PRISMA 2020 Guideline. PROSPERO: CRD42021285412. Pooled odds ratios (ORs) with 95% confidence intervals (CI) were calculated using the random-effects model when at least three comparable outcomes were available. We found no significant difference between quadrivalent live-attenuated intranasal and trivalent inactivated intramuscular (OR = 1.48; 95% CI 0.49-4.45) or between trivalent live-attenuated intranasal and inactivated intramuscular vaccines (OR = 0.77, CI = 0.44-1.34) regarding their efficacy. However, the subgroup analysis of large, multi-center trials indicated that the trivalent live attenuated intranasal influenza vaccine was superior to the trivalent inactivated intramuscular influenza vaccine (12,154 people, OR = 0.50, CI = 0.28-0.88). Only 23 "vaccine-related serious adverse events" were recorded among 17 833 individuals, with no significant difference between methods. The widespread initiation of pediatric national flu vaccination programs prioritizing the live-attenuated intranasal influenza vaccine would be beneficial. Multi-continent, high-quality studies that include children younger than two years old and those living in subtropical and tropical regions are needed to further enhance our understanding.
Keywords: Cost-effectiveness; Flu; Health policy; Pain-free vaccines; Vaccine efficacy; Vaccine safety.
© 2024. The Author(s).
Conflict of interest statement
There is nothing to declare.
Figures
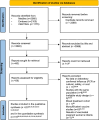


References
-
- Khaled M. Influenza (seasonal). 2024. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). World Health Organization. Accessed 25 Apr 2024.
-
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2023–2024. Pediatrics. 2023;152: e2023063773. - PubMed
-
- Ilya K. Managing seasonal vaccination policies and coverage in the European region. 2014. https://www.who.int/europe/activities/managing-seasonal-vaccination-poli.... WHO Task Force for Global Health. Accessed 16 Apr 2024.
-
- Al Awaidi S, Abusrewil S, AbuHasan M, Akcay M, Aksakal FNB, Bashir U, Elahmer O, Esteghamati A, Gahwagi M, Mirza YK, et al. Influenza vaccination situation in Middle-East and North Africa countries. Report of the 7th MENA Influenza Stakeholders Network (MENA-ISN). J Infect Public Health. 2018;11:845–50. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

