STAT3 blockade ameliorates LPS-induced kidney injury through macrophage-driven inflammation
- PMID: 39367511
- PMCID: PMC11453053
- DOI: 10.1186/s12964-024-01841-1
STAT3 blockade ameliorates LPS-induced kidney injury through macrophage-driven inflammation
Abstract
Background: Signal transducer and activator of transcription 3 (STAT3), a multifaceted transcription factor, modulates host immune responses by activating cellular response to signaling ligands. STAT3 has a pivotal role in the pathophysiology of kidney injury by counterbalancing resident macrophage phenotypes under inflammation conditions. However, STAT3's role in acute kidney injury (AKI), particularly in macrophage migration, and in chronic kidney disease (CKD) through fibrosis development, remains unclear.
Methods: Stattic (a JAK2/STAT3 inhibitor, 5 mg/kg or 10 mg/kg) was administered to evaluate the therapeutic effect on LPS-induced AKI (L-AKI) and LPS-induced CKD (L-CKD), with animals sacrificed 6-24 h and 14 days post-LPS induction, respectively. The immune mechanisms of STAT3 blockade were determined by comparing the macrophage phenotypes and correlated with renal function parameters. Also, the transcriptomic analysis was used to confirm the anti-inflammatory effect of L-AKI, and the anti-fibrotic role was further evaluated in the L-CKD model.
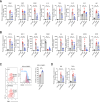
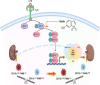
Results: In the L-AKI model, sequential increases in BUN and blood creatinine levels were time-dependent, with a marked elevation of 0-6 h after LPS injection. Notably, two newly identified macrophage subpopulations (CD11bhighF4/80low and CD11blowF4/80high), exhibited population changes, with an increase in the CD11bhighF4/80low population and a decrease in the CD11blowF4/80high macrophages. Corresponding to the FACS results, the tubular injury score, NGAL, F4/80, and p-STAT3 expression in the tubular regions were elevated. STAT3 inhibitor injection in L-AKI and L-CKD mice reduced renal injury and fibrosis. M2-type subpopulation with CD206 in CD11blowF4/80high population increased in the Stattic-treated group compared with that in the LPS-alone group in the L-AKI model. Additionally, STAT3 inhibitor reduced inflammation driven by LPS-stimulated macrophages and epithelial cells injury in the co-culture system. Transcriptomic profiling identified 3 common genes in the JAK-STAT, TLR, and TNF signaling pathways and 11 common genes in the LPS with macrophage response. The PI3K-AKT (IL-6, Akt3, and Pik3r1) and JAK-STAT pathways were determined as potential Stattic targets. Further confirmation through mRNA and protein expressions analyses showed that Stattic treatment reduced inflammation in the L-AKI and fibrosis in the L-CKD mice.
Conclusions: STAT3 blockade effectively mitigated inflammation by retrieving the CD11blowF4/80high population, further emphasizing the role of STAT3-associated macrophage-driven inflammation in kidney injury.
Keywords: AKI; Fibrosis; LPS; Macrophage-driven inflammation; STAT3.
Plain language summary
This study investigated the role of STAT3 in LPS-induced acute kidney injury (AKI) and its prolonged pathophysiological effect. In a mouse model, blocking STAT3 with Stattic reduced inflammation and fibrosis, decreased the levels of inflammatory and extracellular matrix (ECM) substances, reduced the number of certain immune cells (macrophages), and influenced specific genes related to inflammation. The findings suggest that targeting STAT3 is a promising approach to treat AKI and CKD by controlling the inflammation and the immune response as well as ECM accumulation. This study provides novel insights into AKI and CKD progression and will facilitate the development of new treatments for kidney injuries at various stages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

